Zoonotic Helminth Diseases in Dogs and Dingoes Utilising Shared Resources in an Australian Aboriginal Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Dingoes
2.2.1. Dingo Necropsy Samples
2.2.2. Dingo Trapping
2.2.3. Processing Dingoes
2.2.4. GPS Tracking
2.3. Domestic Dogs
2.3.1. Faecal and Tissue Samples
2.3.2. Domestic Dog Tracking
2.4. Parasitology Techniques
2.4.1. Detection of Adult D. immitis antigen
2.4.2. Blood Smears
2.4.3. Faecal Examination
2.4.4. Microscopic Examination
2.5. Data Analysis
2.6. Ethics
3. Results
3.1. Parasite Infections
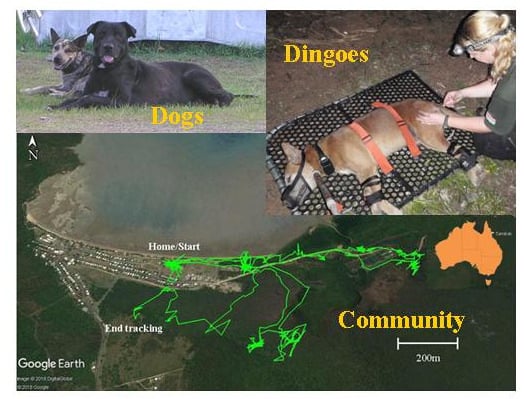
3.2. Home Ranges and Resource Use
4. Discussion
5. Conclusions
List of Abbreviations
| GPS | Global Positioning System |
| WTWHA | Wet Tropics World Heritage Area |
| BCS | Body condition score |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hughes, J.; Macdonald, D.W. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 2013, 157, 341–351. [Google Scholar] [CrossRef]
- Knobel, D.L.; Butler, J.R.; Lembo, T.; Critchlow, R.; Gompper, M.E. Dogs, Disease, and Wildlife; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Gompper, M.E. The Dog-Human-Wildlife Interface: Assessing the Scope of the Problem; Oxford University Press: Oxford, UK, 2014; pp. 9–54. [Google Scholar]
- Butler, J.; Linnell, J.; Morrant, D.; Athreya, V.; Lescureux, N.; McKeown, A. Dog eat dog, cat eat dog: Social-ecological dimensions of dog predation by wild carnivores. In proceeding of Free-Ranging Dogs and Wildlife Conservation; Oxford University Press: Oxford, UK, 2014; pp. 117–143. [Google Scholar]
- Smout, F.; Schrieber, L.; Speare, R.; Skerratt, L. More bark than bite: Comparative studies are needed to determine the importance of canine zoonoses in Aboriginal communities. A critical review of published research. Zoonoses Public Health 2017, 64, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Kurscheid, J.; Jones, M.K.; Gray, D.J.; McManus, D.P. Soil-transmitted helminths in tropical Australia and Asia. Trop. Med. Infect. Dis. 2017, 2, 56. [Google Scholar] [CrossRef] [PubMed]
- Shield, J.; Aland, K.; Kearns, T.; Gongdjalk, G.; Holt, D.; Currie, B.; Provic, P. Intestinal parasites of children and adults in a remote aboriginal community of the Northern Territory, Australia, 1994–1996. West. Pac. Surveill. Response 2015, 6, 44–51. [Google Scholar] [CrossRef]
- Palmer, C.S.; Traub, R.J.; Robertson, I.D.; Hobbs, R.P.; Elliot, A.; While, L.; Rees, R.; Thompson, R. The veterinary and public health significance of hookworm in dogs and cats in Australia and the status of A. ceylanicum. Vet. Parasitol. 2007, 145, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Smout, F.A.; Skerratt, L.F.; Butler, J.R.; Johnson, C.N.; Congdon, B.C.; Thompson, R.A. The hookworm Ancylostoma ceylanicum: An emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health 2017, 3, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Smout, F.A.; Thompson, R.; Skerratt, L.F. First report of Ancylostoma ceylanicum in wild canids. Int. J. Parasitol. Parasites Wildl. 2013, 2, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Bradbury, R.S.; Stevens, M.A.; Haydon, S.R.; Jex, A.R.; Gasser, R.B. Genetic characterization of selected parasites from people with histories of gastrointestinal disorders using a mutation scanning-coupled approach. Electrophoresis 2013, 34, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.M.; Grove, D.I. Experimental infection of humans with Ancylostoma ceylanicum: Clinical, parasitological, haematological and immunological findings. Trop. Geogr. Med. 1986, 38, 38–45. [Google Scholar] [PubMed]
- Hsu, Y.C.; Lin, J.T. Intestinal infestation with Ancylostoma ceylanicum. N. Engl. J. Med. 2012, 366, e20. [Google Scholar] [CrossRef] [PubMed]
- Smout, F.A.; Skerratt, L.F.; Butler, J.R.; Johnson, C.N.; Congdon, B.C. Dingoes (Canis dingo Meyer, 1793) continue to be an important reservoir host of Dirofilaria immitis in low density housing areas in Australia. Vet. Parasitol. 2016, 215, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kochar, A.S. Human pulmonary dirofilariasis: Report of three cases and brief review of the literature. Am. J. Clin. Pathol. 1985, 84, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.; Amaraneni, A.; Singh, S.; Roach, R. Man’s best friend: How humans can develop Dirofilaria immitis infections. ID Cases 2016, 4, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.R.A.; du Toit, J.T.; Bingham, J. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: Threats of competition and disease to large wild carnivores. Biol. Conserv. 2004, 115, 369–378. [Google Scholar] [CrossRef]
- Kennedy, B.; Brown, W.Y.; Vernes, K.; Körtner, G.; Butler, J.R. Dog and cat interactions in a remote Aboriginal community. Animals 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.; Irwin, P.; Lymbery, A.; Thompson, R. The role of companion animals in the emergence of parasitic zoonoses. Int. J. Parasitol. 2000, 30, 1369–1377. [Google Scholar] [CrossRef]
- Polley, L. Navigating parasite webs and parasite flow: Emerging and re-emerging parasitic zoonoses of wildlife origin. Int. J. Parasitol. 2005, 35, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Wilks, K. Sustainable dog health programs are possible: West Australian experiences in remote management and service delivery. In Proceedings of the Conference on Dog Health Programs in Indigenous Communities, Darwin, Australia, August 2000. [Google Scholar]
- Hardaker, J. Making sustainable improvements in animal welfare in remote Indigenous communities. ‘A strategic approach to animal welfare improvement in remote Indigenous communities’. In Proceedings of the AAWS International Conference, Gold Coast, Australia, 31 August–3 September 2008. [Google Scholar]
- WTMA. Wet Tropics Conservation Strategy: The Conservation, Rehabilitation and Transmission to Future Generations of the Wet Tropics World Heritage Area; Wet Tropics Management Authority: Cairns, Australia, 2004. [Google Scholar]
- WTMA. Wet Tropics Management Authority Strategic Plan 2013–18; Wet Tropics Management Authority: Cairns, Australia, 2013. [Google Scholar]
- Australian Bureau of Statistics. ‘Yarrabah (ILOC30201101) Indigenous Profile’. Available online: http://www.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/IARE302011?opendocument (accessed on 8 September 2017).
- Morrant, D.S.; Johnson, C.N.; Butler, J.R.; Congdon, B.C. Biodiversity friend or foe: Land use by a top predator, the dingo in contested landscapes of the Australian Wet Tropics. Austral Ecol. 2017, 42, 252–264. [Google Scholar] [CrossRef]
- Lewis, J.S.; Rachlow, J.L.; Garton, E.O.; Vierling, L.A. Effects of habitat on GPS collar performance: Using data screening to reduce location error. J. Appl. Ecol. 2007, 44, 663–671. [Google Scholar] [CrossRef]
- Atkins, C.E. Comparison of results of three commercial heartworm antigen test kits in dogs with low heartworm burdens. J. Am. Vet. Med. Assoc. 2003, 222, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.M.; Ware, W.A. Canine heartworm disease. Iowa State Univ. Vet. 1989, 51, 7. [Google Scholar]
- Kelly, J. Detection and differentiation of microfilariae in canine blood. Aust. Vet. J. 1973, 49, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, T.; Weinstein, P.; BLOCH, J. Canine filariasis—The influence of the method of treatment on measurements of microfilariae in blood samples. Am. J. Vet. Res. 1963, 24, 395–401. [Google Scholar] [PubMed]
- Stringfellow, G.; Francis, I.; Coroneo, M.; Walker, J. Orbital dirofilariasis. Clin. Exp. Ophthalmol. 2002, 30, 378. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.J.; Inpankaew, T.; Sutthikornchai, C.; Sukthana, Y.; Thompson, R. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet. Parasitol. 2008, 155, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Biocca, E. On Ancylostoma braziliense (de Faria, 1910) and its morphological differentiation from A. ceylanicum (Looss, 1911). J. Helminthol. 1951, 25, 1–10. [Google Scholar] [CrossRef]
- Mohr, C.O. Table of equivalent populations of North American small mammals. Am. Midl. Nat. 1947, 37, 223–249. [Google Scholar] [CrossRef]
- Šlapeta, J.; Dowd, S.E.; Alanazi, A.D.; Westman, M.E.; Brown, G.K. Differences in the faecal microbiome of non-diarrhoeic clinically healthy dogs and cats associated with Giardia duodenalis infection: Impact of hookworms and coccidia. Int. J. Parasitol. 2015, 45, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Wallner, J. The use of a coproantigen ELISA to determine the prevalence of Echinococcus granulosus in pig-hunting dogs in Townsville/Thuringowa. Master’s Thesis, James Cook University, Townsville, Australia, 1999. [Google Scholar]
- Gillespie, S.; Bradbury, R.S. A survey of intestinal parasites of domestic dogs in Central Queensland. Trop. Med. Infect. Dis. 2017, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Prociv, P.; Croese, J. Human eosinophilic enteritis caused by dog hookworm Ancylostoma caninum. Lancet 1990, 335, 1299–1302. [Google Scholar] [CrossRef]
- Prociv, P.; Croese, J. Human enteric infection with Ancylostoma caninum: Hookworms reappraised in the light of a ‘new’ zoonosis. Acta Trop. 1996, 62, 23–44. [Google Scholar] [CrossRef]
- Kelly, J. Canine Parasitology; Post-Graduate Foundation in Veterinary Science: Sydney, Australia, 1977. [Google Scholar]
- Dunsmore, J.; Shaw, S. Clinical Parasitology of Dogs; University of Sydney, Post Graduate Foundation in Veterinary Science: Sydney, Australia, 1990. [Google Scholar]
- Traversa, D. Are we paying too much attention to cardio-pulmonary nematodes and neglecting old-fashioned worms like Trichuris vulpis? Parasites Vectors 2011, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Geldhof, P.; Albonico, M.; Ame, S.M.; Bethony, J.M.; Engels, D.; Mekonnen, Z.; Montresor, A.; Sopheak, H.; Tchuem-Tchuenté, L.-A. Molecular speciation of soil-transmitted helminths egg isolates collected during six drug efficacy trials in endemic countries. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 657. [Google Scholar]
- Areekul, P.; Putaporntip, C.; Pattanawong, U.; Sitthicharoenchai, P.; Jongwutiwes, S. Trichuris vulpis and T. trichiura infections among schoolchildren of a rural community in northwestern Thailand: The possible role of dogs in disease transmission. Asian Biomed. 2010, 4, 49–60. [Google Scholar] [CrossRef]
- Coman, B. Helminth parasites in the dingo and feral dog in Victoria with some notes on the diet of the host. Aust. Vet. J. 1972, 48, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Feldman, G.J.; Parker, H.W. Visceral larva migrans associated with the hypereosinophilic syndrome and the onset of severe asthma. Ann. Intern. Med. 1992, 116, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Overgaauw, P.A.; van Knapen, F. Dogs and nematode zoonoses. In Dogs, Zoonoses and Public Health; Macpherson, C.N.L., Meslin, F.X., Wandeler, A.I., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 213–256. [Google Scholar]
- Smout, F.; James Cook University, Townsville, Australia. Personal communication, 201.
- Speare, R. An Overview of Canine Parasitic Diseases, Their Treatment and Prevention. Available online: https://www.amrric.org/sites/default/files/Canine_Parasitic_Diseases.pdf (accessed on 5 October 2018).
- Brown, B.; Copeman, D.B. Zoonotic importance of parasites in wild dogs caught in the vicinity of Townsville. Aust. Vet. J. 2003, 81, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Chappell, C.; Enos, J.; Penn, H. Dipylidium caninum, an under-recognized infection in infants and children. Pediatr. Infect. Dis. J. 1990, 9, 745–747. [Google Scholar] [PubMed]
- Morrant, D.S.; Wurster, C.M.; Johnson, C.N.; Butler, J.R.A.; Congdon, B.C. Prey use by dingoes in a contested landscape: Ecosystem service provider or biodiversity threat? Ecol. Evolut. 2017, 7, 8927–8935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Allen, L.; Goullet, M. Encroachment of Echinococcus granulosus into urban areas in eastern Queensland, Australia. Aust. Vet. J. 2008, 86, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Banks, D.; Copeman, D.; Skerratt, L.; Molina, E. Echinococcus granulosus in northern Queensland. Aust. Vet. J. 2006, 84, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Fleming, P.; Corbett, L.K.; Harden, R.; Thomson, P. Managing the Impacts of Dingoes and other Wild Dogs; Bureau of Rural Sciences: Canberra, Australia, 2001.
- Burger, A.; Knowles, P. Fraser Island; Rigby: Adelaide, Australia, 1976. [Google Scholar]
- Møller, A.P. Evidence of larger impact of parasites on hosts in the tropics: Investment in immune function within and outside the tropics. Oikos 1998, 82, 265–270. [Google Scholar] [CrossRef]
- Meek, P.D. The movement, roaming behaviour and home range of free-roaming domestic dogs, Canis lupus familiaris, in coastal New South Wales. Wildl. Res. 1999, 26, 847–855. [Google Scholar] [CrossRef]
- Dürr, S.; Ward, M.P. Roaming behaviour and home range estimation of domestic dogs in Aboriginal and Torres Strait Islander communities in northern Australia using four different methods. Prev. Vet. Med. 2014, 117, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, J.; Körtner, G.; Ballard, G.; Fleming, P.J.; Brown, W.Y. Effects of sex and reproductive state on interactions between free-roaming domestic dogs. PLoS ONE 2014, 9, e116053. [Google Scholar] [CrossRef] [PubMed]



| (% Prevalence) | Necropsy Dingo † | Collared Dingo * | Total Dingoes | Necropsy Dog † | Collared Dog * | Dog Scats * | Total Dogs |
|---|---|---|---|---|---|---|---|
| (n = 27) | (n = 12) | (n = 39) | (n = 28) | (n = 7) | (n = 50) | (n = 85) | |
| Nematoda | |||||||
| Ancylostoma caninum | 27 (100) (91–100) § | 8/8 (100) 4NI (78–100) | 35/35 (100) (93–100) | 27 (96) (86–100) | 7 (100) (77–100) | 44 (88) (78–98) | 78 (92) (85–98) |
| Ancylostoma ceylanicum | 3 (11) (0–24) | 1/8 (13) 4NI (0–38) | 4/35 (11) (0–23) | 0 | 0 | 0 | 0 |
| Toxocara canis | 12 (44) (27–62) | 4 (33) (9–58) | 16 (41) (26–56) | 13 (46) (29–64) | 2 (28.6) (0–58) | 10 (20) (9–31) | 25 (29) (20–39) |
| Trichuris vulpis | 1 (4) (7–14) | 1 (8) (0–28) | 2 (5) (0–14) | 22 (79) (63–94) | 2 (28.6) (0–58) | 17 (34) (21–47) | 41 (48) (38–59) |
| Dirofilaria immitis | 10 (37) (20–55) | 8 (67) (43–91) | 18 (46) (31–61) | 0 | NI | NI | 0 |
| Cestoda | |||||||
| Dipylidium caninum | 1 (4) (7–14) | 0 | 1 (3) (0–10) | 20 (71) (55–88) | 0 | 0 | 20 (24) (14–33) |
| Spirometra erinacei | 12 (44) (27–62) | 6 (50) (25–75) | 18 (46) (31–61) | 0 | 0 | 3 (6) (0–14) | 3 (4) (0–8) |
| Dingo/Dog | Sex | Mass (kg) [BCS] | Duration of Tracking (days) | Home Range (km2) | Location |
|---|---|---|---|---|---|
| Dingo | |||||
| TD01 | Male | 27 [5] | 79 | 107.3 * | Walsh’s Pyramid |
| TD02 | Male | 21 [5] | 195 | 76.8 * | Mount Peter |
| TD03 | Male | 21.5 [4] | 120 | 34.5 * | Glen Boughton |
| TD04 | Female | 17 [3] | 150 | 57.1 * | Walsh’s Pyramid |
| TD06 | Female | 13 [3] | 122 | 6.9 * | Old Smithfield |
| TD07 | Female | 9 [3] | 101 | 8.1 * | Old Smithfield |
| TD08 | Female | 13 [3] | 171 | 79.0 * | Walsh’s Pyramid |
| TD09 | Female | 15 [4] | 202 | 85.9 * | Glen Boughton |
| TD10 | Female | 14.5 [4] | 96 | 26.1 * | Walsh’s Pyramid |
| TD11 | Female | 14 [3] | 17 | 5.1 | Yarrabah |
| TD12 | Male | 14 [4] | 58 | 6.2 | Yarrabah |
| Mean | 44.8 (±11.38) | ||||
| Dog | |||||
| CD01 | Female (desexed) | 14 [5] | 67 | 2.4 | Yarrabah |
| CD02 | Male | 30 [5] | 28 | 2.7 | Yarrabah |
| CD03 | Male | 33 [5] | 80 | 2.6 | Yarrabah |
| CD04 | Male | 32 [5] | 15 | 0.6 | Yarrabah |
| CD05 | Male | 7 [5] | 26 | 2.2 | Yarrabah |
| CD06 | Male | 38 [5] | 65 | 0.4 | Yarrabah |
| CD07 | Female | 30 [5] | 27 | 5.1 | Yarrabah |
| Mean | 2.3 (±0.59) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smout, F.A.; Skerratt, L.F.; Johnson, C.N.; Butler, J.R.A.; Congdon, B.C. Zoonotic Helminth Diseases in Dogs and Dingoes Utilising Shared Resources in an Australian Aboriginal Community. Trop. Med. Infect. Dis. 2018, 3, 110. https://doi.org/10.3390/tropicalmed3040110
Smout FA, Skerratt LF, Johnson CN, Butler JRA, Congdon BC. Zoonotic Helminth Diseases in Dogs and Dingoes Utilising Shared Resources in an Australian Aboriginal Community. Tropical Medicine and Infectious Disease. 2018; 3(4):110. https://doi.org/10.3390/tropicalmed3040110
Chicago/Turabian StyleSmout, Felicity A., Lee F. Skerratt, Christopher N. Johnson, James R. A. Butler, and Bradley C. Congdon. 2018. "Zoonotic Helminth Diseases in Dogs and Dingoes Utilising Shared Resources in an Australian Aboriginal Community" Tropical Medicine and Infectious Disease 3, no. 4: 110. https://doi.org/10.3390/tropicalmed3040110
APA StyleSmout, F. A., Skerratt, L. F., Johnson, C. N., Butler, J. R. A., & Congdon, B. C. (2018). Zoonotic Helminth Diseases in Dogs and Dingoes Utilising Shared Resources in an Australian Aboriginal Community. Tropical Medicine and Infectious Disease, 3(4), 110. https://doi.org/10.3390/tropicalmed3040110