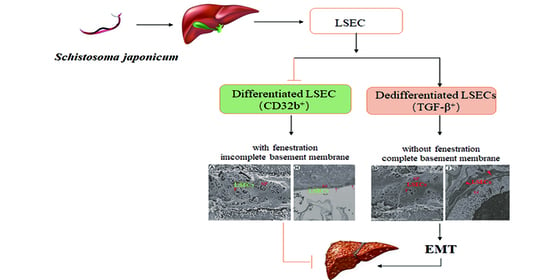
Pathological Changes in Hepatic Sinusoidal Endothelial Cells in Schistosoma japonicum-Infected Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Parasites
2.3. Reagents
2.4. Infection and Cell Isolation
2.5. Flow Cytometry
2.6. Electron Microscopy
2.7. Reverse Transcription Quantitative PCR
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. The Changes in the Proportion of LSECs in Mice Infected with S. japonicum
3.2. Changes in the Number of Fenestrations of LSEC after Infection
3.3. The Changes of EMT in LSECs after Infection with S. japonicum
3.4. EMT and Liver Fibrosis in the Liver after Infection with S. japonicum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LoVerde, P.T. Schistosomiasis. Adv. Exp. Med. Biol. 2019, 1154, 45–70. [Google Scholar] [PubMed]
- Zhang, L.J.; Xu, Z.M.; Yang, F.; Dang, H.; Li, Y.L.; Lv, S.; Chao, C.L.; Xu, J.; Li, S.Z.; Zhou, X.L. Endemic status of schistosomiasis in People’s Republic of China in 2020. Chin. J. Schistosomiasis Control 2021, 33, 225–233. [Google Scholar]
- Wang, W.; Bergquist, R.; King, C.H.; Yang, K. Elimination of schistosomiasis in China: Current status and future prospects. PLoS Negl. Trop. Dis. 2021, 15, e0009578. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.F.; Zhu, L.Q.; Li, Y.L.; Zhang, L.J.; Xue, J.B.; Xia, S.; Lv, S.; Xu, J.; Li, S.Z. Identification of the high-risk area for schistosomiasis transmission in China based on information value and machine learning: A newly data-driven modeling attempt. Infect. Dis. Poverty 2021, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.C.; Bezerra, F.S.M.; Colley, D.G.; Fleming, F.M.; Homeida, M.; Kabatereine, N.; Kabole, F.M.; King, C.H.; Mafe, M.A.; Midzi, N.; et al. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet. Infect. Dis. 2022, 22, e327–e335. [Google Scholar] [CrossRef]
- Qin, Z.Q.; Xu, J.; Feng, T.; Lv, S.; Qian, Y.J.; Zhang, L.J.; Li, Y.L.; Lv, C.; Bergquist, R.; Li, S.Z.; et al. Field Evaluation of a Loop-Mediated Isothermal Amplification (LAMP) Platform for the Detection of Schistosoma japonicum Infection in Oncomelania hupensis Snails. Trop. Med. Infect. Dis. 2018, 3, 124. [Google Scholar] [CrossRef] [Green Version]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Lafoz, E.; Ruart, M.; Anton, A.; Oncins, A.; Hernández-Gea, V. The endothelium as a driver of liver fibrosis and regeneration. Cells 2020, 9, 929. [Google Scholar] [CrossRef] [Green Version]
- Deleve, L.D.; Wang, X.; Guo, Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 2008, 48, 920–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Liu, X.; Zhang, M.; Niu, J.Q. Liver sinusoidal endothelial cells are implicated in multiple fibrotic mechanisms. Mol. Biol. Rep. 2021, 48, 2803–2815. [Google Scholar] [CrossRef] [PubMed]
- Hammoutene, A.; Rautou, P.E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019, 70, 1278–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribera, J.; Pauta, M.; Melgar-Lesmes, P.; Córdoba, B.; Bosch, A.; Calvo, M.; Rodrigo-Torres, D.; Sancho-Bru, P.; Mira, A.; Jiménez, W.; et al. A small population of liver endothelial cells undergoes endothelial-to-mesenchymal transition in response to chronic liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G492–G504. [Google Scholar] [CrossRef] [Green Version]
- Kochan, K.; Kus, E.; Filipek, A.; Szafrańska, K.; Chlopicki, S.; Baranska, M. Label-free spectroscopic characterization of liver liver sinusoidal endothelial cells (LSECs) isolated from the murine liver. Analyst 2017, 142, 1308–1319. [Google Scholar] [CrossRef]
- Deol, A.K.; Fleming, F.M.; Calvo-Urbano, B.; Walker, M.; Bucumi, V.; Gnandou, I.; Tukahebwa, E.M.; Jemu, S.; Mwingira, U.; Alkohlani, A.; et al. Schistosomiasis—Assessing progress toward the 2020 and 2025 global goals. N. Engl. J. Med. 2019, 381, 2519–2528. [Google Scholar] [CrossRef]
- Carbonell, C.; Rodríguez-Alonso, B.; López-Bernús, A.; Almeida, H.; Galindo-Pérez, I.; Velasco-Tirado, V.; Marcos, M.; Pardo-Lledías, J.; Belhassen-García, M. Clinical spectrum of schistosomiasis: An update. J. Clin. Med. 2021, 10, 5521. [Google Scholar] [CrossRef]
- McManus, D.P. The search for a schistosomiasis vaccine: Australia’s contribution. Vaccines 2021, 9, 872. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef]
- Zhu, J.F.; Xu, Z.P.; Chen, X.J.; Zhou, S.; Zhang, W.W.; Chi, Y.; Li, W.; Song, X.; Liu, F.; Su, C. Parasitic antigens alter macrophage polarization during Schistosoma japonicum infection in mice. Parasit Vectors 2014, 7, 122. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Fan, Y.; Guo, D.Y.; Xu, B.; Shi, X.Y.; Li, J.T.; Duan, L.F. Study on the relationship between hepatic fibrosis and epithelial-mesenchymal transition in intrahepatic cells. Biomed. Pharmacother. 2020, 129, 110413. [Google Scholar] [CrossRef]
- Villesen, I.F.; Daniels, S.J.; Leeming, D.J.; Karsdal, M.A.; Nielsen, M.J. Review article: The signalling and functional role of the extracellular matrix in the development of liver fibrosis. Aliment. Pharmacol. Ther. 2020, 52, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhai, S.M.; Huang, H.F.; Zeng, Z. A balance mediated by liver sinusoidal endothelial cells on liver regeneration and fibrosis. Chin. J. Hepatobiliary Surg. 2021, 27, 545–548. [Google Scholar]
- Hu, J.; Srivastava, K.; Wieland, M.; Runge, A.; Mogler, C.; Besemfelder, E.; Terhardt, D.; Vogel, M.J.; Cao, L.J.; Korn, C.; et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science 2014, 343, 416–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.D.; Xu, M.Y.; Cai, X.B.; Qu, Y.; Li, Z.H.; Lu, L.G. Myofibroblastic transformation of rat hepatic stellate cells: The role of Notch signaling and epithelial-mesenchymal transition regulation. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4130–4138. [Google Scholar] [PubMed]
- Ikegami, T.; Zhang, Y.; Matsuzaki, Y. Liver fibrosis: Possible involvement of EMT. Cells Tissues Organs 2007, 185, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Cicchini, C.; Amicone, L.; Alonzi, T.; Marchetti, A.; Mancone, C.; Tripodi, M. Molecular mechanisms controlling the phenotype and the EMT/MET dynamics of hepatocyte. Liver Int. 2015, 35, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, J.; Srinivasan, H.; Mani, K.P. Molecular mechanism involved in epithelial to mesenchymal transition. Arch. Biochem. Biophys. 2021, 710, 108984. [Google Scholar] [CrossRef]
- Choi, S.S.; Diehl, A.M. Epithelial-to-mesenchymal transitions in the liver. Hepatology 2009, 50, 2007–2013. [Google Scholar] [CrossRef] [Green Version]
- Hammoutene, A.; Biquard, L.; Lasselin, J.; Kheloufi, M.; Tanguy, M.; Vion, A.C.; Mérian, J.; Colnot, N.; Loyer, X.; Tedgui, A.; et al. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J. Hepatol. 2020, 72, 528–538. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; Qurashi, M.; Shetty, S. The role of sinusoidal endothelial cells in the axis of inflammation and cancer within the liver. Front. Physiol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.P.; Chen, Z.Y.; Ye, L. Research progress of molecular mechanism in epithelial-mesenchymal transformation (EMT) in hepatic fibrosis. J. Med. Theory Pract. 2019, 18, 2886–2888. [Google Scholar]
- Bravo, M.; Raurell, I.; Hide, D.; Fernández-Iglesias, A.; Gil, M.; Barberá, A.; Salcedo, M.T.; Augustin, S.; Genescà, J.; Martell, M. Restoration of liver sinusoidal cell phenotypes by statins improves portal hypertension and histology in rats with NASH. Sci. Rep. 2019, 9, 20183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruart, M.; Chavarria, L.; Campreciós, G.; Suárez-Herrera, N.; Montironi, C.; Guixé-Muntet, S.; Bosch, J.; Friedman, S.L.; Garcia-Pagán, J.C.; Hernández-Gea, V. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J. Hepatol. 2019, 70, 458–469. [Google Scholar] [CrossRef]
- Su, T.H.; Kao, J.H.; Liu, C.J. Molecular mechanism and treatment of viral hepatitis-related liver fibrosis. Int. J. Mol. Sci. 2014, 15, 10578–10604. [Google Scholar] [CrossRef]
- Carson, J.P.; Ramm, G.A.; Robinson, M.W.; McManus, D.P.; Gobert, G.N. Schistosome-induced fibrotic disease: The role of hepatic stellate cells. Trends. Parasitol. 2018, 4, 524–540. [Google Scholar] [CrossRef]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. Wiley Interdiscip. Rev. Syst. Biol. Med. 2021, 13, e1499. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y. Gut-liver-axis: Barrier function of liver sinusoidal endothelial cell. J. Gastroenterol. Hepatol. 2021, 36, 2706–2714. [Google Scholar] [CrossRef]
- Wu, X.; Shu, L.; Zhang, Z.; Li, J.; Zong, J.; Cheong, L.Y.; Ye, D.; Lam, K.S.L.; Song, E.; Wang, C.; et al. Adipocyte fatty acid binding protein promotes the onset and progression of liver fibrosis via mediating the crosstalk between liver sinusoidal endothelial cells and hepatic stellate cells. Adv. Sci. 2021, 8, e2003721. [Google Scholar] [CrossRef]
- Di Martino, J.; Mascalchi, P.; Legros, P.; Lacomme, S.; Gontier, E.; Bioulac-Sage, P.; Balabaud, C.; Moreau, V.; Saltel, F. Actin depolymerization in dedifferentiated liver sinusoidal endothelial cells promotes fenestrae re-formation. Hepatol. Commun. 2018, 3, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Shi, Z.; Zhao, Y.; Meng, X.; Zhao, S.; Zheng, L.; Han, X.; Hu, Z.; Yao, Q.; Lin, H.; et al. LncRNA Airn maintains LSEC differentiation to alleviate liver fibrosis via the KLF2-eNOS-sGC pathway. BMC Med. 2022, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis-from immunopathology to vaccines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Primer Sequence (5′→3′) |
|---|---|
| GAPDH | F:CATCACTGCCACCCAGAAGACTG |
| R:ATGCCATGAGCTTCCCGTTCAG | |
| E-cadherin | F:GGTCATCAGTGTGCTCACCTCT |
| R:GCTGTTGTGCTCAAGCCTTCAC | |
| VE-cadherin | F:GAACGAGGACAGCAACTTCACC |
| R:GTTAGCGTGCTGGTTCCAGTCA | |
| Zonula occludens1 (ZO1) | F:GTTGGTACGGTGCCCTGAAAGA |
| R:GCTGACAGGTAGGACAGACGAT | |
| Fibronectin (FN) | F:GGTCCTCTCCTTCCATCTCCTTAC |
| R:GGACCCCTGAGCATCTTGAGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Wu, X.; Zhou, H.; Hu, Y.; Cao, J. Pathological Changes in Hepatic Sinusoidal Endothelial Cells in Schistosoma japonicum-Infected Mice. Trop. Med. Infect. Dis. 2023, 8, 124. https://doi.org/10.3390/tropicalmed8020124
Jiang T, Wu X, Zhou H, Hu Y, Cao J. Pathological Changes in Hepatic Sinusoidal Endothelial Cells in Schistosoma japonicum-Infected Mice. Tropical Medicine and Infectious Disease. 2023; 8(2):124. https://doi.org/10.3390/tropicalmed8020124
Chicago/Turabian StyleJiang, Tingting, Xiaoying Wu, Hao Zhou, Yuan Hu, and Jianping Cao. 2023. "Pathological Changes in Hepatic Sinusoidal Endothelial Cells in Schistosoma japonicum-Infected Mice" Tropical Medicine and Infectious Disease 8, no. 2: 124. https://doi.org/10.3390/tropicalmed8020124
APA StyleJiang, T., Wu, X., Zhou, H., Hu, Y., & Cao, J. (2023). Pathological Changes in Hepatic Sinusoidal Endothelial Cells in Schistosoma japonicum-Infected Mice. Tropical Medicine and Infectious Disease, 8(2), 124. https://doi.org/10.3390/tropicalmed8020124