1. Introduction
Laser beam sources are widely used in current production applications. For material processing, the efficiency of the laser beam process is determined by the absorption of the radiation by the irradiated material. The absorptivity of a material depends amongst other things on the laser beam wavelength, the material, and its surface topography. Measuring this absorptivity was first carried out using a calorimetric approach with thermocouples [
1]. Those studies have shown the strong influence of coatings on absorptivity, which is challenging for laser-based surface heat treatments such as laser beam hardening. The absorption of steel during irradiation with laser radiation of different wavelengths was analyzed by Dausinger and Shen [
2]. They found that the absorption of 1 µm laser radiation of iron for heating up to the melting point decreases with increasing temperatures, whereas CO
2 laser radiation increases with higher temperatures.
Furthermore, the influence of the surface structure was investigated. On the one hand, it is generally known that rougher surfaces have a higher absorptivity [
3]. Therefore, metals can be grinded or particle blasted as a pre-treatment to increase the absorptivity [
4]. However, on the other hand, several studies have shown that conventional roughness values like
Ra,
Rq, and
Rz are not suitable for directly predicting the absorptivity of a metal surface. For example, specimens having comparable roughness values showed differing absorptivity [
3]. In addition, peak values of absorptivity were determined between 2 and 4 µm for the maximum surface height (
Rz) [
3]. Instead of using these conventional roughness values, it was suggested to use the mean slope of the surface profile [
5].
Another approach to characterizing the effect of roughness on the absorptivity of radiation was proposed by Agababov [
6]. A form factor was defined that divided the area size of a smooth surface corresponding to the area of the measuring field by the surface of the actual roughness geometry. According to radiation measurements with a pyrometer, the absorptivity of radiation was increasing with lower form factors. In addition, it was found that mean square roughness values did not satisfactorily reflect the absorption changes, explained by the missing consideration of the shape, structure, and distribution of the roughness [
6].
The surface structure of steel sheets can also be modified by oxidation which is caused by heating under an oxygen-containing atmosphere. Early investigations showed the influence of lower temperatures up to 650 °C and holding times of 0.5 s to 200 days on the oxidation of several steels [
7]. Based on time and temperature parameters, the colors straw, blue, and brown can be predicted to appear on the surface. For the stainless steel 1.4301, investigations of the oxidation process showed that the annealing color represents the oxide layer thickness [
8]. After heating at 650 °C for 7 min, the surface turned blue and no change in the oxide layer thickness was observed for up to 10 min. That blue color was determined to be 130 nm to 140 nm thick [
9]. These annealing colors can also be used for controlled surface coloring (e.g., [
10]).
During the oxidation of the material 304L, mixed iron–chromium oxides (spinel) are formed at room temperature, whereas iron-rich oxides result after a 2 min oxidation at 600 K without shielding gas [
11]. Further investigations mention chromium oxide growth for the heating of AISI 304 at 850 °C [
12]. These findings were confirmed for the oxidation of stainless steel in air plasma [
13]. In the range of 900 to 1100 °C, the material 1.4301 mainly forms FeCr
2O
4 spinel [
14]. Generally, the oxidation behavior of stainless steel can be described with the parabolic law [
15]. However, investigations of diffusion processes in vacuum indicate the formation of a healing layer at the grain boundaries which modifies the rate law [
16]. This is more pronounced the higher the chromium content and the annealing times; no deviation from the parabolic law was measured for up to 30 min at 700 °C [
16]. Additionally, the oxidation rate was found to be dependent on the grain size, with finer grains having a higher oxidation rate [
17].
The oxidation of steel increases absorptivity, which is the reason why surfaces for laser heating are often coated with graphite to achieve a constant absorption during the heating process [
4]. Oxidation was also found to be a dominant factor for absorptivity changes in investigations on the hardening of low-alloy steel [
18]. However, the extent of absorptivity changes caused by short-term laser beam irradiation has been insufficiently studied.
In order to describe the relationship between material loads and material modifications, a process signature was defined [
19]. That universal description provides information about the resulting surface and boundary zone properties, whereby the manufacturing process is irrelevant. With a process signature, a correlation between the load of a process and the resulting modification is drawn which enables the generation of surface integrity states based on knowledge [
20]. This also enables substitutions of manufacturing processes by obtaining corresponding process signatures.
The generation of a process signature was carried out for a thermal-induced white layer formation in 42CrMo4 steel [
21]. It was shown that a certain temperature field diameter represents a process signature for laser-chemical machining in different media, argon and sodium nitrate in this case. That characteristic value had not yet been described for the absorptivity changes caused by thermal influences on steel sheet surfaces.
3. Materials and Methods
3.1. Materials
For these investigations, three different steel sheets were used, namely, the stainless steel X5CrNi18-10 (1.4301), the spring steel C75S+LC (1.1248), and the hot work tool steel X40CrMoV5-1 (1.2344). The sheet thickness was 3.0 mm for the stainless steel (1.4301) and the spring steel (1.1248). The tool steel specimens (1.2344) were machined from a block to a thickness of 4.0 mm. The materials were cut into specimens of 50 × 76 mm
2. Compositions of the steels used are listed in
Table 1.
3.2. Experimental Setup and Program
Each specimen was characterized prior to and after a specific temperature treatment with a multi‑kW laser. This characterization included roughness and absorption measurements.
A disc laser (Trumpf TruDisk 12002, TRUMPF GmbH + Co. KG, Ditzingen, Germany; wavelength: 1030 nm, BPP: 8 mm*mrad, NA: 0.1) and a laser optic (Trumpf BEO D70); collimation length 200 mm, focal length 300 mm, Rayleigh length: 2.298 mm) were used for the laser beam heating setup. The maximum laser power was limited to 8 kW due to the limits of the optical components. Tryouts were done with a 50 mm defocused laser beam, resulting in a measured spot size of 6.3 mm.
In order to ensure a constant temperature during the desired processing time, a closed‑loop control for the frequency of laser pulses was used. The laser power was set to 500 W for all experiments. The pulse duration was 6 ms with a controlled frequency in the range of 0 to 100 Hz.
Several time–temperature curves were obtained in order to investigate the effects of different temperature impacts as well as holding times on the absorption of the sheet surface. Laser processing was carried out with varying temperature target values from 700 to 1200 °C for the stainless steel (1.4301) and 700 to 900 °C for the materials 1.1248 and 1.2344. The duration of laser heating was varied between 2 and 34 s. Additionally, a heating‑up time of 2 s was applied for each target temperature. Additionally, increasing temperature intervals (700 °C → 800 °C → 900 °C) with constant (2 s each, 5 s each, 10 s each) as well as varying time steps (e.g., 2 s → 5 s → 10 s) were investigated.
Every parameter set was carried out three times. Processing was carried out at ambient atmosphere and without shielding gas.
3.3. Temperature Measurements and Analyses
During the punctual laser irradiation of the steel sheet surface, the surface temperature was measured with a digital two-color pyrometer (LumaSense Technologies IMPAC IGAR 12‑LO, LumaSense Technologies GmbH, Frankfurt, Germany). The measuring range extended from 500 to 2200 °C.
For the comparability of time and temperature effects, a characteristic value was calculated which characterized the time–temperature curve including the heating and cooling phases. Such a parameter has already been defined as
temperature‑compensated time (
tc) for the description of diffusion processes in powder metallurgy [
22], as shown in Equation (1):
This calculation contains the prefactor of the diffusion coefficient
D0, the activation energy
Qeff, the Boltzmann constant
k, as well as the time–temperature distribution
Ta(
t). The value is indicated in the unit
m2, which at first seems unusual. However, the
temperature‑compensated time (
tc) can be illustrated by considering the equation for the penetration depth
x of a diffusion process:
The
temperature‑compensated time can be determined by numerical integration of the temperature measurement signal and literature values for the activation energy
Qeff and the prefactor of the diffusion coefficient
D0. During the oxidation of the material 1.4301 in air, mainly FeCr
2O
4 spinel is formed in the temperature range of 900 to 1100 °C [
14]. The literature values used for the diffusion of oxygen in γ-iron also differ only slightly from the characteristic values used for the diffusion of oxygen in chromium [
23]. Therefore, the literature values for an oxygen diffusion into iron is used in this case [
23].
In
Figure 1, the integrated area for calculating the
temperature‑compensated time is marked in blue.
3.4. Absorption Measurement
The absorption of each specimen was characterized using an integrating sphere (Ø 100 mm; BaSO
4 coated; three ports) setup (see
Figure 2). Irradiation of the specimen was carried out using a laser diode (Lumics LU1030M300, Lumics GmbH, Berlin, Germany) with a wavelength of 1030 nm and a maximum power of 300 mW. This laser source was connected to the top port of the sphere with a Thorlabs CFC-2X-B collimator (Thorlabs GmbH, Dachau/Munich, Germany) with a focal length of 2 mm and a numerical aperture (NA) of 0.5. The measuring laser beam full-angle divergence with this collimator is indicated as 0.123°, resulting in an output waist diameter of 0.38 mm that is considered to be the spot size on the sheet surface. Metal sheets were applied at the second port of the sphere. This knife-edge port had an opening with a diameter of 5 mm. Reflected radiation from the specimen was measured at the third port with a Si-photodiode (Thorlabs FDS100, Thorlabs GmbH, Dachau/Munich, BY, Germany, rise time = 10 ns). The measured radiation is assumed to be homogenized due to the coating of the sphere which is highly diffuse reflective. Therefore, measurements of the photo diode are considered to be representative. Measurements of an Nd:YAG mirror (Thorlabs NB07-K14, Thorlabs GmbH, Dachau/Munich, Germany) were used for calibration of the system. The reflectance of unpolarized laser radiation with a wavelength of 1030 nm and an irradiation angle of 8° was 99.89369% according to its datasheet. Measurements of this mirror were set to this value.
3.5. Roughness Measurement
For analyzing the roughness, the surface of each specimen was measured using a color 3D laser scanning microscope Keyence VK 9700 (KEYENCE DEUTSCHLAND GmbH, Neu-Isenburg, Germany). In this case, a 50× lens with an aperture of 0.55 was used, resulting in a measurement field of 184.9 × 328.7 µm2. Measured data were filtered with a Gaussian filter, an S-filter of 0.8 µm, and an L‑filter of 0.5 mm. Positioning of the roughness measuring was set concentrically to the laser interaction spot on the sheet surface.
For the characterization of the surface topography, surface roughness values for the arithmetical mean height (Sa), the root mean square height (Sq), and the maximum height (Sz) were determined according to ISO 25178. Additionally, the hybrid roughness value Sdq, determining the root mean square gradient of slopes, and the topography factor surface/area were calculated. For this topography factor, the surface of the roughness profile is divided by the measurement field size mentioned above.
3.6. SEM Analyses
For high magnification imaging of the surface modifications resulting from laser irradiation, the scanning electron microscope (SEM) Zeiss EVO MA10 (Carl Zeiss Microscopy GmbH, Jena, Germany) was used. This device was equipped with a LaB6-cathode. For the investigations of surface modifications, secondary electron (SE) detection was used.
4. Results
For the analysis of the effects of short-term heating by laser irradiation, the initial states of the analyzed values must be measured. Therefore, the mean values of surface topography and absorption measurements of all untreated specimens are listed in
Table 2 for each material.
During the irradiation process, no shielding gas was used. Therefore, laser irradiation resulted in a clearly visible color change which is attributed to oxidation.
Irradiations of the stainless steel sheets (1.4301) did not have a significant effect on the roughness values
Sa and
Sq. For higher
temperature‑compensated times above 1000 µm
2, a slight reduction of these values can be observed (see
Figure 3). This tendency is even more pronounced for the value
Sz.
A similar progression can be observed for the hybrid roughness value
Sdq (see
Figure 4). The topography factor
surface/area is slightly increased for increasing
temperature‑compensated times.
For the spring steel investigated in this study (1.1248), low irradiations, characterized by a low
temperature‑compensated time, led to a smoother surface in terms of lower
Sa and
Sq values (see
Figure 5). Higher roughness values can only be identified for intense irradiations with
temperature‑compensated times over 250 µm
2. The maximum heights (
Sz) of the laser‑heated surfaces are almost identical to the initial state.
Both the changes in the hybrid roughness value
Sdq and in the topography factor surface/area are increased with increasing
temperature‑compensated times (see
Figure 6). However, it is noticeable that a reduction of these values can be observed for low
temperature‑compensated times. Changes in the topography factor
surface/area are positive for
temperature‑compensated times over 50 µm
2.
Sdq values are closer to the initial state the more intense is the heating.
The roughness changes of the hot work tool steel (1.2344) induced by laser beam heating are shown in
Figure 7. The mean values
Sa and
Sq are not significantly changed by laser irradiation, although there is an outlier with a decrease of the roughness and simultaneously high standard deviation at a
temperature‑compensated time of 61.84 µm
2. However, changes in the maximum height (
Sz) of irradiated surfaces show a tendency to decrease with increasing
temperature‑compensated times.
Short‑term heating of the hot work tool steel (1.2344) seems to have no significant influence on the hybrid roughness value
Sdq or the topography factor
surface/area (see
Figure 8). A significant increase of the topography factor
surface/area can only be reported for a
temperature‑compensated time of 320.9 µm
2.
Overall, changes in the surface roughness values or the topography factor indicated by short‑term heating with a laser beam do not indicate a clear tendency, although increased absorptivity values were measured after heating for all materials (see
Figure 9 and
Figure 10). Generally, maximum surface height value changes (
Sz) are more scattered compared to the changes in the mean roughness values
Sa and
Sq (see
Figure 9).
Influences of changes in the hybrid roughness value
Sdq and the topography factor
surface/area on the absorptivity are shown in
Figure 10. For the stainless steel (1.4301) and the tool steel (1.2344), both increasing and decreasing values for
Sdq and
surface/area result in increased absorptivity. A tendency cannot be observed.
For the spring steel (1.1248), increasing values for Sdq and surface/area correlate with increasing absorptivity. However, lower absorptivity changes around 20 % relate to decreased values of Sdq and surface/area.
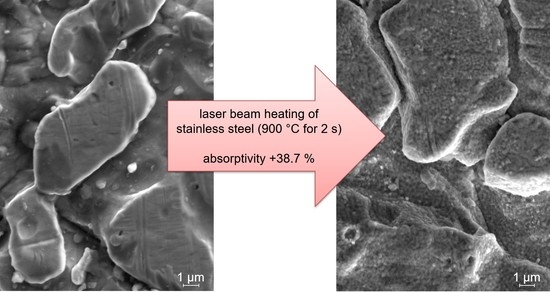
The influence of short-term heating on the surface structure at a sub-micrometer scale was investigated with SEM pictures (see
Figure 11). Compared to the initial state, small particles can be observed on the surface of the material, which are considered to be oxides. Roughness of the specimen illustrated in
Figure 11 is nearly not affected. Only the maximum height of the surface (
Sz) is increased by 40%. The reduction of the topography factor
surface/area, however, is below 10%.
On the contrary, absorptivity is significantly increased by the laser beam heating of the specimen illustrated in
Figure 11. In this case, heating with the parameters of a temperature of 900 °C for 2 s resulted in an absorptivity increase of 38.7%.
The absorptivity changes of each material for all parameter sets are shown in
Figure 12. The value of the
temperature‑compensated time is used to illustrate the influence of several time-temperature distributions on the absorptivity changes. In general, increasing
temperature‑compensated times increase the absorptivity. However, for the stainless steel (1.4301),
temperature‑compensated times over 323.2 µm
2 do not further increase the absorptivity compared to lower heating durations. It can be observed that even intense irradiations do not increase the total absorptivity over 87.5% in this study. This behavior indicates a state of saturation.
Changes in the absorptivity of the tool steel (1.2344) due to short-term heating are generally much more reactive. The described saturation level is already reached for lower temperature‑compensated times of 9.88 µm2. Further heating slightly further increases the absorptivity.
The spring steel (1.1248) shows comparable absorptivity changes as the stainless steel (1.4301) for temperature‑compensated times of 26.7 µm2 and higher. For lower temperature‑compensated times, the change in absorptivity is more than double the change that occurs on the stainless steel (1.4301).
In order to describe the increasing absorptivity of the stainless steel (1.4301) by increasing
temperature‑compensated times, only short-term heating durations leading to increasing absorptivity up to a total absorption value of 80% are illustrated in
Figure 13. It can be determined that the increasing absorptivity can be approximated by a logarithmic function.
5. Discussion
In this study, different alloys show comparable effects. The magnitude of absorptivity changes caused by short-term laser heating of the spring steel (1.1248) is comparable to that of the stainless steel (1.4301). The absorptivity changes of the tool steel (1.2344) reacts much faster to laser heating, which results in higher absorptivity changes when comparing the same irradiation conditions.
Investigations of the roughness prior to and after the laser heating indicate that absorptivity changes are not attributed to roughness changes induced by the short-term heating process (see
Figure 9). The surface roughness values, like the conventional values (see Reference [
3]), are not considered to be suitable to describe or predict the absorption of a metal surface, at least for the investigated steels. Instead of these roughness values, Bergström proposed considering the mean slope of a surface profile for the description of absorptivity [
5]. However, the hybrid roughness value
Sdq, which is the root mean square gradient and therefore close to the proposal of Bergström, seems not to be suitable for absorptivity predictions, at least for the stainless steel (1.4301) and the tool steel (1.2344). For the spring steel (1.1248), there is a certain dependency. However, on the one hand, intensive irradiations causing high absorptivity changes result in negligible changes in the
Sdq value (see
Figure 10). On the other hand, low irradiation results in a reduction of the
Sdq value. Therefore, further additional indicators are required to predict the absorptivity changes of the spring steel (1.1248) with this hybrid roughness factor.
Experiments by Agababov showed a higher degree of blackness for lower roughness factors, which was defined as measurement area divided by the roughness surface [
6]. According to this, absorptivity should be increased with higher values of the topography factor
surface/area. This topography factor increases for all materials used in this study when intense laser beam heating, in terms of a high
temperature‑compensated time, was carried out. However, absorptivity changes of 1.4301 and 1.2344 do not correlate with this topography factor (see
Figure 10). For the steel 1.1248, higher topography factors led to higher absorptivity values, but lower laser irradiations showed reduced values for the topography factor (see
Figure 6). Therefore, the theoretical relationship drawn by Agababov might be correct but is obviously counteracted by another effect.
A more detailed analysis of the surface changes induced by laser heating illustrates a structural change in the sub‑micrometer range (see
Figure 11). This indicates an oxide layer growth that is clearly affecting the absorptivity. However, this oxide layer is too small to have an influence on roughness values. Therefore, oxide layer thickness is assumed to be much smaller than the hundreds of nanometers quoted in investigations carried out by Junqueira et al. [
9] who observed a significant difference due to the roughness of the oxide film. Investigations of oxide formations on the surface of stainless steel (see Reference [
12]) report larger structures for similar temperatures but rather long heating times of 50 hours compared to the 2 seconds pictured in
Figure 11.
On the one hand, roughness modifications of short-term laser heating did not correlate with the absorptivity changes found for irradiations of the steels mentioned. On the other hand, oxidation of the surface occurred due to the absence of shielding gas. Additionally, significant absorptivity changes were measured after laser irradiation. Therefore, the author ascribes oxidation as the dominating effect for increasing absorptivity, which confirms Pantsar and Kujanpää who found this for low-alloy steel [
18]. Furthermore, a saturation effect can be observed especially for the stainless steel (1.4301). Since the parameter of the
temperature‑compensated time is based on material-specific diffusion parameters, there should be a causal relationship to the absorptivity changes for all specimens up to the state of saturation. This assumption is supported by the visualization of absorptivity changes of the stainless steel (1.4301) sheets with absorption values below 80% after laser irradiation (see
Figure 13). The increasing absorption behavior can be described by a logarithmic regression function. The coefficient of determination is 0.92 for this correlation function, which underlines the significance of the
temperature‑compensated time at least for the stainless steel (1.4301).
Overall, the short-term heating via laser beam can be characterized by introducing the
temperature‑compensated time. With this characteristic value, absorption changes can be described for a certain material. The parameters of heating temperature and heating duration are combined into one characteristic value, whereby high temperatures with short durations result in the same absorptivity change as low temperatures with longer durations if the
temperature‑compensated time is the same. Therefore, the
temperature‑compensated time is a suitable value to represent a process signature, as defined by Brinksmeier et al. [
19], for absorption changes caused by short-term heating.