1. Introduction
Due to the rapid industrialization and urban development across the globe, the usage of petroleum-based inert plastics has been drastically increased [
1,
2,
3] in daily life, the biomedical field, agriculture, and in the food industry [
4,
5] owing to their low cost, lightweight [
6], strength to weight ratio, easy processability, durability [
6,
7,
8], improved barrier properties, and heat stability [
9]. However, the tremendous increase in the usage and production of petroleum-based plastic materials has resulted in vast amounts of plastic waste on the land sites [
1,
10] since they take 100–450 years to degrade naturally [
11,
12,
13,
14]. Apart from the above, it is a well-known fact that non-degradable polymers are derived from petroleum and its allied components [
2]. Consequently, the depletion of petroleum resources can occur since the natural resources take millions of years to form and are finite in quantity [
15].
Although, at present, numerous ways of managing polymeric wastes are introduced, including incineration, recycling, and energy recovery systems, incineration in landfilling sites may lead to the release of heat and unacceptable emissions of harmful compounds, such as greenhouse gases and volatile compounds, into the atmosphere [
16,
17]. In contrast, the recycling process is rarely used due to the consumption of a considerable amount of thermal energy, the complexity of design, and relatively high cost, reducing the process sustainability [
9,
18]. Thus, waste plastics are eventually destined to be burnt or buried in landfill sites [
9]. Therefore, disposal problems, strict regulations on plastic use, new criteria for a cleaner and safer environment [
13,
19], and the global shortage of petroleum resources have driven the development of biodegradable and renewable materials [
11,
20,
21] as alternatives to replace or reduce synthetic plastics.
Generally, single-use plastic materials used in food packaging applications, personal care products, agricultural purposes, and fishing equipment are released directly to the environment [
22]. Therefore, it is conclusive that recycling is not practicable and economical for single-use plastics [
23]. Furthermore, due to the depletion of petroleum resources and environmental concerns, the development of environmentally friendly alternatives to meet the ever-increasing demand for single-use plastics materials, known as ‘green materials’ [
24], has become vital importance. Therefore, many approaches are currently underway to utilize biodegradable polymers as alternatives for non-biodegradable synthetic polymers, minimizing environmental and other commercial issues [
25]. Interestingly, starch, as a packaging material, has attracted much attention both in academia as well as the industry [
26] owing to its relative abundance, renewability [
26,
27], biodegradability [
27], low cost, easy handling [
4], and the capability of dissolving in water [
28].
Despite starch’s inherent superior properties, native starch cannot be utilized in practical applications due to its ever-increasing brittleness with time in the absence of a suitable plasticizer, poor processability and storage stability, and low mechanical and thermal properties [
23,
29,
30]. Though starch is plasticized to obtain thermoplastic starch (TPS) to improve its properties, TPS alone also cannot be used as a substitute for petroleum-based inert plastic materials due to its poor mechanical and thermal properties, water sensitivity, deterioration of mechanical properties during the exposure to humid environments, poor barrier properties, and plasticizer migration [
23,
29,
30,
31,
32,
33,
34]. Moreover, packaging materials composed entirely of starch lack the strength and rigidity to withstand mechanical stresses. Hence, the solution is to blend starch with a robust support base [
1] to widen its range of applications [
4]. Therefore, the blending of TPS with synthetic biodegradable polymers has become an attractive pathway to overcoming the major drawbacks of TPS while achieving specific requirements of an application [
23].
It is well-known that starch is a hydrophilic polymer since each starch monomer consists of three free hydroxyl groups in its chemical structure [
23]. In contrast, synthetic polymers are hydrophobic and thermodynamically immiscible with hydrophilic starch. Therefore, simple mixing of these two polymer components may result in phase separation, phase incompatibility, and poor mechanical properties [
30,
35]. However, the mechanical properties of polymer blends can be enhanced by incorporating a suitable compatibilizing agent during the blend preparation [
31], and this process of stabilizing polymer blends is called ‘compatibilization’ [
36].
Though the immiscible polymer blends are compatibilized using suitable compatibilizing agents, the properties of the blends play a significant role in determining the appropriate amount of the compatibilizer to be incorporated and the polymer ratio in the blends, which provides the required properties for a specific application. Despite the fact that blend features mainly depend on the properties of the individual polymer components present in the blend, the morphology of the blend film is the major factor for producing polymer blends with enhanced properties [
23]. Apart from the morphology, it is said that the crystallinity of the polymer phases also plays a significant role in performing the properties of a polymer blend [
24]. Though the phase morphology of starch-based blends is coarse due to starch’s high molecular weight, strong hydrogen bonding, and hydrophilic nature [
22], a fine morphology is required for the enhanced mechanical properties of a blend [
22,
24].
Therefore, it is conclusive that the mechanical properties of a certain polymer blend reflect the compatibility between the two polymer phases [
37]. Usually, the elongation at break and the toughness of a polymer blend are governed by the domain size of the dispersed phase and the interfacial adhesion between the matrix and the dispersed phase [
38]. Apart from the mechanical properties, thermal and barrier properties of a particular blend are also important parameters to be altered during packaging applications [
39]. It is a commonly known fact that water is a plasticizer for starch. Water absorption of starch-based blends leads to increased mobility, and the starch molecules tend to reorganize and aggregate themselves. Therefore, any improvement in water resistance in these blends is of paramount importance [
40]. Besides, melt flow index (MFI) for a particular polymer blend is also a good guideline during processing [
41].
Recent advances have prompted this review on incorporating different compatibilizing agents for starch/synthetic biodegradable polymer blends to improve essential properties associated with packaging applications. Therefore, this review focuses on starch/synthetic biodegradable polymer blends for sustainable packaging applications. Most importantly, the effects of various compatibilization processes on the thermo-mechanical, physical, and functional properties of different polymer blends have also been reviewed.
2. Non-Degradable Petroleum-Based Plastic Materials
With the diverse range of innovations in plastic production, they have been employed in many sectors, including packaging, automotive, construction, healthcare, and electronics. Therefore, due to the incremental usage of plastic, plastic waste generation has also increased over the last few decades [
12,
13,
42]. Moreover, the global consumption of plastic products has significantly increased to around 400 million tons [
8]. Almost all the plastic materials are made up of polyolefins such as polypropylene (PP), polycarbonate (PC), polyvinyl chloride (PVC), polyethylene (PE), and polystyrene (PS). These synthetic or non-biodegradable polymers are derived from petroleum-based materials [
15].
Table 1 depicts the widely used non-biodegradable petroleum-based plastic materials, applications, and their contribution to the solid waste, and recycling percentages. PET is one of the most consumed plastics among those plastic waste due to its intrinsic properties, including scalability, lightweight, and pressure resistivity [
43].
As can be evidenced from
Table 1, PET has been widely used in the global plastic market with the second highest recyclability. High-density polyethylene (HDPE) is a long linear polymer chain with a high crystalline structure and low branching, providing high strength properties. HDPE is considered the third-largest type of plastic found in municipal solid waste contributing nearly 17.6% to plastic waste, as shown in
Table 1. Moreover, low-density polyethylene (LDPE) is widely used in squeezing bottles. Although LDPE is reusable, they are not always recycled. Therefore, LDPE waste has accumulated to become the second-largest plastic waste in municipal solid waste after PP [
44]. PVC is a soft, flexible plastic-type, recycled less than 1% after use (see
Table 1). PP is a saturated polymer with a linear hydrocarbon chain with good chemical and heat resistance while being tough and light in weight. According to
Table 1, in the US, 3% of PP is recycled. Furthermore, PS is a cheap, lightweight plastic made up of styrene monomers obtained from liquid petrochemicals. The structure of PS consists of a long hydrocarbon chain containing a phenyl group attached to each carbon atom [
43].
Table 1 justifies the importance of non-biodegradable plastics in day-to-day lives and current issues associated with the recycling and disposal of synthetic plastics. As shown in
Table 1, the highest percentage of plastics is sent for landfilling, occupying a significant space on the land. In Europe, approximately 38%, 26%, and 36% of plastic waste are generally sent for landfilling, recycling, and energy recovery through utilization, respectively [
43].
3. Biodegradable Polymers
Biodegradable polymers are used for various packaging, building materials, hygiene products, and medical applications, due to their intriguing properties, such as durability, easy processing, and relatively low-cost manufacturing [
45]. Biodegradable polymers can be classified according to chemical composition, synthesis method, processing method, economic importance, and applications [
46]. Generally, these polymers degrade into final products like water, carbon dioxide, minerals, and intermediate products (biomass and humid materials) in a natural environment [
47]. Moreover, biodegradable polymers can be derived from renewable or petroleum resources [
23].
Biodegradable polymers are generally categorized into two major groups as natural and synthetic biopolymers based on their origin. Furthermore, according to Avérous and Pollet [
48] and Ghanbarzadeh and Almasi [
46], biodegradable polymers can be classified into three major groups based on their origin, namely (i) natural polymers, (ii) synthetic polymers, and (iii) modified natural polymers (see
Figure 1) [
46,
48].
Figure 1 shows the schematic representation of biodegradable polymers based on their source of origin.
Interestingly, biodegradable polymers offer tremendous potential uses in many exciting applications, such as drug delivery, tissue engineering, gene therapy, regenerative medicine, temporary implantable devices, food containers, soil retention sheeting, agricultural mulch film, waste bags, and packaging materials. [
23,
25,
49]. Among these diverse applications, the development of packaging materials to address the ever-increasing demand has been significant since plastic materials used in food packaging and other personal care products are mostly single-use. Moreover, natural biodegradable polymers, including chitosan, cellulose, chitin, cyclodextrin, and starch, have recently captured attention due to their low toxicity, biocompatibility, and biodegradability [
50,
51,
52,
53].
4. Starch
Starch, which is the second most abundant [
54,
55] biopolymer after cellulose [
23] and one of the low-cost polysaccharides [
56], is a common constituent that can be found in all organs of higher plants. Starch is the major polysaccharide chain that stores carbohydrates. Apart from the higher plants, starch can be found in mosses, ferns, protozoa, algae and bacteria. Starch is widely present in green plants and every type of tissue, including leaves, fruits, roots, stems, shoots, and pollen grains [
57]. The main botanical origins of starch production are maize, cassava, wheat, and potato, respectively [
58]. Starch content in potato tubers, maize endosperms, sweet potato, and cassava and yam roots varies from 65% to 90% of the total dry weight. Starch consists of two types of polysaccharides, typically known as linear amylose and branch amylopectin [
59], as shown in
Figure 2. Amylose is composed of D-glucose units linked by 1–4 glycosidic bonds (see
Figure 2a) while amylopectin is composed of poly glucose units linked by 1–4 and 1–6 glycosidic bonds [
60] (see
Figure 2b). Generally, the amount of amylose and amylopectin varies according to the source of origin [
57].
Starch was first used extensively in the plastic industry as a filler [
61] to produce eco-friendly and low-cost plastic materials [
23,
62]. However, as a solution for minimizing non-biodegradable plastic materials, starch is used in its plasticized form, known as thermoplastic starch (TPS), to compound with other synthetic biodegradable polymers, subsequently reducing the ‘white pollution’ and carbon footprint [
22,
62].
5. Thermoplastic Starch (TPS)
Native starch is not suitable for direct practical applications due to brittleness, low processability and storage stability [
23,
30,
63,
64]. Though native starch is considered as a non-plasticized material due to the intra- and intermolecular hydrogen bonds between the hydroxyl groups of starch molecules, starch can be converted into a continuous polymeric entangled phase by mixing with aqueous or non-aqueous plasticizers, including glycerol, glycol, xylitol, sorbitol, sugars, ethanolamine, urea, formamide, acetamide etc. [
23]. During the thermoplastic process, in the presence of plasticizers, a semi-crystalline granule of starch is transformed into a homogeneous material via hydrogen bond cleavage between starch molecules, leading to the loss of crystallinity, reduced glass transition temperature of starch, and improved chain flexibility. Finally, petroleum-like TPS polymer with melt processing ability is formed [
23,
65]. Therefore, when a plasticizer is added to starch, it becomes easier to process than native starch and converts into a moldable material. Most importantly, the properties of TPS highly depend on the amount and the type of plasticizer added [
22,
23].
Among different plasticizers, glycerol is considered the most widely used due to its low cost, non-toxicity, and high boiling point. According to the literature, both native and modified starch is used in TPS preparation [
66]. It is reported that TPS from starch acetates presents enhanced mechanical properties and reduced hydrophilicity. Moreover, TPS prepared from dialdehyde starch exhibit reduced glass transition temperature, increased mechanical properties and reduced hydrophilicity and water vapor permeability [
23]. Although starch is plasticized in the presence of different plasticizers, TPS alone cannot be used as a substitute for petroleum-based inert plastic materials. Therefore, TPS is blended with another biodegradable polymer [
23]. The major reasons for blending TPS with synthetic biodegradable polymers are to reduce the cost [
22,
23,
37,
40], improve biodegradation rate [
40,
67], maintain biocompatibility and renewability [
22], and achieve acceptable levels of physio-mechanical properties [
56]. Therefore, the combination of these two polymer components provides synergetic effects [
37].
6. Synthetic Biodegradable Polymers
TPS is widely blended with hydrophobic biodegradable polymers, such as polylactic acid (PLA), polycaprolactone (PCL), polyhydroxybutyrate (PHB), poly(3-hydroxybutryate-
co-3-hydroxyvalerate) (PHBV), polybutylene succinate (PBS), and poly (butylene adipate-
co-terephthalate) (PBAT) (see
Figure 3) [
23].
Figure 3 shows the chemical structures of common synthetic biodegradable polymers. Usually, reactive melt blending is carried out by incorporating an appropriate compatibilizing agent to increase TPS content without severely impacting the mechanical properties while linking the two immiscible polymers through covalent bonds and enhancing the interfacial adhesion among them. This strong interfacial adhesion leads to an effective stress transfer between the two polymer phases [
68].
Polycaprolactone, commonly known as PCL (see
Figure 3a), is a typical aliphatic polyester that plays a significant role in packaging and medical applications [
39,
69]. PCL is linear, hydrophobic, partially crystalline, and can be slowly utilized by microorganisms. Moreover, its physical properties and commercial availability make it very attractive as a substitute for non-biodegradable polymers for commodity applications [
70]. Due to the properties such as biodegradability, biocompatibility, non-toxicity, and resistance to water, oil, solvent, and chlorine, PCL is highly considered in industries. However, the main limitation of PCL is the low melting temperature which can be overcome by blending with another polymer [
70,
71]. Among possible blending methods, PCL with starch has become a commodity that reduces moisture susceptibility and enhances thermal and mechanical properties [
39].
Polyvinyl alcohol, known as PVA (see
Figure 3c), has become one of the widely used and available biodegradable synthetic polymers [
72,
73], which offers exciting properties, such as good processability, water solubility [
72], higher thermal stability [
27], good barrier properties [
11], easy processability, chemical resistance [
19], better mechanical properties [
12,
14,
19,
26], non-toxicity [
26,
74], biocompatibility, and better film-forming and adhesive properties [
26]. In addition, PVA is one of the few synthetic polymers produced via hydrolysis of polyvinyl acetate [
72], a non-petroleum route [
75].
Polybutylene succinate (PBS) is a biodegradable polyester, consisting of bio-based carbon content between 35% and 50% [
67], see
Figure 3e. PBS exhibits promising properties, such as excellent impact strength, melt processability, biodegradability, high flexibility, good thermal stability, and good chemical resistivity. Moreover, these properties are quite similar to those of polyethylene [
37,
41,
76].
Polylactic acid (PLA) is derived through the bacterial fermentation of annually recyclable plant-based carbohydrates, such as starch, sugarcane, and bagasse,
Figure 3b. However, the commercial production of PLA involves condensation polymerization followed by ring-opening polymerization of lactic acid. PLA plays a significant role in the packaging industry since it possesses impressive mechanical, chemical, and gas barrier properties, along with biocompatibility and odorless characteristics [
33,
77,
78,
79].
Poly(3-hydroxybutrate-
co-3-hydroxyvalerate), commonly known as PHBV (see
Figure 3f), is one of the commercially available biodegradable synthetic polymers produced as a reserve material by numerous microorganisms under limited concentrations of essential nutrients, such as nitrogen or phosphorus, and excess carbon source [
80]. Moreover, PHBV is a thermoplastic linear aliphatic polyester produced via copolymerization of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid. Although PHBV is a biodegradable, non-toxic, biocompatible plastic capable of serving as a good alternative for many non-biodegradable polymers, its high melting point (>170 °C), high relative crystallinity, brittleness, and narrow processing window have limited its applications [
80,
81].
Poly(butylene adipate-
co-terephthalate), also known as PBAT (see
Figure 3g), is generally marketed as a fully biodegradable alternative to low-density polyethylene, consisting of similar properties, including flexibility and resilience, allowing it to be used during the production of plastic bags and wraps. Moreover, PBAT is a synthetic aliphatic-aromatic co-polyester often blended with TPS to improve the drawbacks of plasticized starch, reduce the cost, improve the biodegradability, and enhance the mechanical properties and dimensional stability [
82,
83,
84,
85,
86].
Polyhydroxyalkanoate (PHA) is a bio-based aliphatic polyester produced by commonly found microorganisms as an energy storage mechanism. Polyhydroxybutyrate (PHB) is a well-known member of the PHAs family [
87], see
Figure 3d. Although PHB has received much attention due to its renewability, better ultra-violet resistivity, non-toxic nature, biocompostability under both aerobic and anaerobic conditions, PHB is not widely applied alone in industrial applications due to its high cost, poor processability, and brittleness [
22,
88].
8. Preparation Methods of Starch Blends
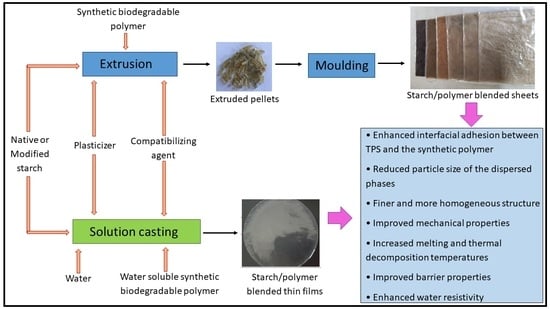
Different types of starch-based blend preparation methods have been adopted by different researchers, such as solution casting, extrusion, injection moulding, compression moulding, and hot-pressing.
Mani et al. prepared compatibilized starch/PCL blends using a co-rotating twin-screw extruder, while the samples for testing were prepared by either compression moulding or injection moulding [
36]. The same method for preparing starch/PCL blends was followed by Ortega-Tora and coworkers [
32]. Moreover, Yin et al. [
24] prepared compatibilized starch/PBS blends following the same method [
24]. Li et al., and Ren et al., prepared starch/PBAT blends and starch/PLA blend sheets using the same method [
33,
89]. Moreover, the same technique was followed by Magalhaes and Andrade [
40] and Ma et al. [
22] for preparing starch/PHBV, starch/PLA and starch/PHB blends.
Kim and coworkers used melt mixing with a Brabender mixer and subjected the blended samples to be moulded via hot-pressing [
38]. The same procedure was followed by Wang et al. [
90] and Liu et al. [
91] for preparing starch/PLA blends [
90,
91]
Both Singh et al., and Sugih and coworkers prepared starch/PCL blends using a mechanical kneader on a two-roll mill, and then the molten composite materials were moulded to investigate the effect of compatibilization [
35,
70]. Furthermore, Akrami et al. [
92] investigated the effect of compatibilization of starch/PLA blended sheets by initially melt mixing with a Brabender mixer, followed by compression moulding [
92]. Chen et al., explored the compatibilization effect of starch/PCL blended sheets by mixing the components in an internal mixer and then by hot-pressing [
29]. A similar procedure was followed by Collazo-Bigliardi et al. [
93] to prepare starch/PLA blends [
93].
Fahrngruber et al. [
67] examined the effect of compatibilization of the starch/PBS blends by preparing the blends via extrusion using a co-rotating twin-screw extruder and subsequently preparing the flat films using a small-scale flat film extrusion line [
67].
Starch/PVA blended films have been prepared by solution casting [
94,
95] to investigate the effect of different compatibilization techniques [
96]. The composite films containing PVA/starch/carbon nanotubes were prepared by solution mixing and casting by Jose et al. [
56]. The exact process for starch/PVA thin film preparation was followed by Gupta et al. [
11] and Widiarto [
11,
97].
9. Synthesis of Compatibilizers
Mani et al. investigated the effect of starch grafted PCL (Starch-g-PCL) as the compatibilizer for starch/PCL blends. Herein, the compatibilizer was synthesized via two steps, namely (i) the isocyanate-terminated PCL was prepared by reacting terminal hydroxyl groups of PCLs with diisocyanate, then (ii) isocyanate-terminated PCL was grafted onto starch through the introduction of urethane links [
36]. The authors also reported the effect of polyacrylic acid (PAA) grafted PCL (PAA-g-PCL) synthesized as a compatibilizer for starch/PCL blended films via the copolymerization of a macromonomer named PCL acrylate and acrylic acid [
38]. Sugih and coworkers investigated the effect of two different types of compatibilizers for starch/PCL blended sheets, namely (i) PCL grafted glycidyl methacrylate (PCL-g-GMA) and (ii) PCL-g-diethyl maleate (PCL-g-DEM), by reacting glycidyl methacrylate (GMA) or diethyl maleate (DEM) with low molecular PCL and benzoyl peroxide (BPO) as the radical initiator [
35]. Ortega-Tora and coworkers [
32] carried out a study to overcome the incompatibility between starch and PCL by synthesizing two different compatibilizing agents: PCL
MG where polar functional groups were chemically grafted on hydrophobic PCL chains by inserting maleic anhydride (MA) and GMA molecules, and (ii) PCL
G by grafting only GMA onto PCL [
32]. Lopez and coworkers developed starch grafted PCL (St-g-PCL) as a compatibilizer for starch/PCL blends. The St-g-PCL copolymer was obtained under vacuum by employing low doses of Co γ-radiation [
39].
Yin et al. [
24] used MA-g-PBS or rPBS (maleic anhydride grafted PBS) as an interfacial compatibilizing agent to overcome the incompatibility between both starch and PBS [
24]. Furthermore, Suchao-in et al. synthesized starch grafted PBS (Starch-g-PBS) as a compatibilizer to enhance the properties of starch/PBS blends. Herein, the synthesis route during the preparation of starch-g-PBS was carried out according to
Figure 5a using different molar ratios of PBS to starch for 0.63, 1, 1.5, 2.2, 3.5, and 6 mmol of dicyclohexylcarbodiimide (DCC) [
37].
Akrami et al. [
92] studied the effect of maleic anhydride grafted poly-ethylene glycol grafted starch (mPEG-g-St) as a compatibilizer on mechanical properties and biodegradability in TPS/PLA blend composites. In this study, the synthesis of the compatibilizer was undertaken via two steps. As the initial step, the grafting reaction between MA and PEG (4:40
w/
w ratio) was accomplished via melt mixing in a flask at 130 °C with 2 h agitation. Secondly, starch particles (56 wt%) were added to the mixture of PEG and MA, and the reaction was continued for another 2 h at 150 °C [
92]. Another study was carried out to explore the effect of PLA grafted glycidyl methacrylate (GPLA-x) as the compatibilizing agent on the morphological, thermal, mechanical and medium resistance properties of PLA/TPS. The graft copolymer GPLA-x, which contains a hydrophilic chain, was synthesized from glycidyl methacrylate monomer by free radical initiated (benzoyl peroxide initiator) grafting PLA using a melt polymerization reaction [
91].
Wootthikanokkhan and coworkers conducted a study to examine the effect of polylactic acid grafted maleated thermoplastic starch (PLA-g-MTPS) as a compatibilizer in PLA/TPS blends. The PLA-g-MTPS copolymers were prepared by reacting maleic anhydride with TPS, then grafting the maleated TPS with PLA using Luperox101 0.25–1.0 phr (parts per hundred resin) as the initiator [
98], see
Figure 5b.
11. Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications
Compared to current global plastic production, it has been predicted that plastic packaging will increase by 2–3-fold in 2030 and 2050, respectively. Moreover, around 26% of the overall plastic production has been used for packaging applications [
116]. Therefore, replacing the inert petroleum-based plastic materials with “green plastics” has been a timely matter. However, biodegradable polymers are restricted for some applications due to their relatively high production cost, comparatively lower mechanical and thermo-mechanical properties, and water sensitivity compared to certain non-degradable commodity polymers. Thus, numerous biodegradable polymer blends have been developed to overcome these limitations. Currently, Europe is leading the movement in advancing biodegradable polymer blends for packaging across the globe [
117].
Although most carbohydrate polymers are widely utilized as food, the use of carbohydrates in other applications has gained significant attraction over the last few years. This could be ascribed to the wide availability and renewable nature of many carbohydrates, including starch. Interestingly, according to the European Bioplastics market, starch-based blends have captured 18.7%, thus increasing the market for bioplastics. In contrast, starch-based blends have found applications in short lifespan service and flexible and rigid packaging applications owing to their attractive properties, such as biodegradability and gas barrier properties.
Some of the prominent applications of starch composites and blends that exist in the current market are as follows:
- (i)
Loose fill packaging: For the production of loose-fill packages using starch-based products, corn, wheat, hydroxypropylated high amylose corn starch containing a small number of additives, including PVA, glycerol, polyethylene glycol (PEG), or silicon dioxide, and methyl-acrylate grafted corn starch is used (see
Table 11) [
118].
- (ii)
Starch-polyester films: Starch/PCL film composites and blends are currently used in the market as compost bags (see
Table 11) [
118].
Some of the commercially available starch-based blends for packaging applications are depicted in
Table 11.
12. Challenges and Future Perspectives for the Development of Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications
Due to the increasing market of starch blends as rigid and flexible packaging materials, it is vital synthesizing biodegradable materials with optimum performances comparable to those of conventional inert plastics. From a cost and practicality point of view, it is preferable that the starch-based blends contain a high percentage of starch. However, remarkable and faster growth might be expected for the products synthesized using 100% biodegradable polymers, including PLA and PVA, due to significant hurdles existing during the incorporation of high amounts of starch (>25–30 wt%) without compromising material properties.
Although a smaller amount of compatibilizing agent is sufficient to obtain better mechanical properties, surface properties, and thermal properties, the performance of the non-biodegradable polymers is still better than those of starch-based blends. Despite the advantages of biodegradable polymers, mechanical performance is a significant factor determining the materials’ ability to process and manufacture at a large scale and apply in different packaging fields. The barrier performances also play a vital role in maintaining the shelf-life of the packaged products. However, starch can be considered efficient in gas barrier properties but not effective in water barrier properties. Interestingly, in some cases, the compatibilized starch/synthetic biodegradable polymer blends exhibited superior mechanical and oxygen barrier performances compared to conventional inert plastic materials.
A wide range of physical, chemical, and other modification techniques can be applied to improve the properties of the starch blends. For instance, the esterification of starch with fatty acids seems to be a significant modification that improves the hydrophobicity and thermo-plasticity, thus increasing the usefulness of this polysaccharide in packaging production. Moreover, the water barrier properties of starch-based blends need to be improved to compete with conventional plastic. Usually, the porosity of a certain material reflects its ability to uptake moisture. Hence, the porosity of the starch-based blends should be estimated prior to their applications [
119,
120,
121]. Furthermore, antimicrobial activity, sealing properties and food spoilage ability are imperative for food packaging materials. Therefore, those properties of starch/polymer blend before using as food packaging materials.
The source of starch used for blending also plays a significant role. For example, Morgan and Choct [
122] reported that cassava starch is more suitable to produce TPS owing to its remarkable properties, such as higher clarity, low glass transition temperature, and good gel stability [
122]. According to Gunawardene et al., the gelatinization temperature of extracted cassava starch was reported to be 43.27 °C, that is comparatively lower than other starch types [
123].
Over the past few years, cassava cultivation has been drastically increased due to its easy-growing nature at the expense of minimum labor and fertilization, higher yield, and comparatively higher stability against diseases and pests. The authors also reported that cassava cultivation is abundant in many countries due to its ability to grow in any soil. Most importantly, the cassava harvesting area and production have increased by 91.7% and 67.82%, respectively, during the past 30 years, and this is a considerable improvement when compared with other food crops, such as maize, rice, wheat, millet, potato, barley, oats, and sweet potatoes [
124]. Therefore, for further improvement in the cassava value chain, the productivity and yield of cassava should be increased.
Undoubtedly, the research on the compatibilization of starch/synthetic biodegradable polymer blends will continue to rise in the near future. Future research should focus on starch-based blends that possess better water barrier properties, antibacterial properties, and sealing properties containing starch or modified starch for industrial-scale packaging applications.