Chitosan-Based Hierarchical Scaffolds Crosslinked with Genipin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
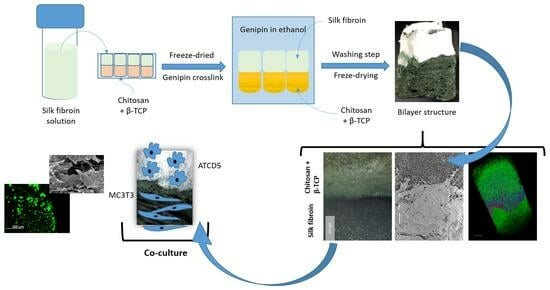
2.2. Production of the CHT/β-Tricalcium Phosphate Layer
2.3. Production of Silk Layer
2.4. Construction of Bilayered Structure
2.5. Determination of Crosslinking Degree
3. Characterization of the Scaffolds
3.1. Scanning Electron Microscopy (SEM)
3.2. Micro-Computed Tomography
3.3. Optical Microscopy
3.4. Mechanical Tests
3.5. Fourier-Transform Infrared Spectroscopy
3.6. Bioactivity Assay
3.7. Swelling Test
3.8. In Vitro Enzymatic Degradation
3.9. Cell Assays
3.9.1. Cell Seeding
3.9.2. Cytotoxicity
3.9.3. Cell Viability
3.9.4. Live–Dead Assay
3.9.5. Cell Proliferation
3.9.6. ALP Activity
3.9.7. Cell Morphology
3.10. Statistical Analysis
4. Results and Discussion
4.1. Morphological Features
4.2. Mechanical Properties
4.3. Structural Features
4.4. Bioactivity Behavior
4.5. Swelling and In Vitro Enzymatic Degradation
4.5.1. Swelling
4.5.2. In Vitro Enzymatic Degradation
4.6. Biological Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morouço, P.; Fernandes, C.; Lattanzi, W. Challenges and Innovations in Osteochondral Regeneration: Insights from Biology and Inputs from Bioengineering toward the Optimization of Tissue Engineering Strategies. J. Funct. Biomater. 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.R.; Carvalho, M.R.; Oliveira, J.M.; Reis, R.L. Tissue Engineering Strategies for Osteochondral Repair. In Osteochondral Tissue Engineering: Challenges, Current Strategies, and Technological Advances; Oliveira, J.M., Pina, S., Reis, R.L., San Roman, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 353–371. [Google Scholar]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef] [PubMed]
- Piaia, L.; Silva, S.S.; Gomes, J.M.; Franco, A.R.; Fernandes, E.M.; Lobo, F.C.M.; Rodrigues, L.C.; Leonor, I.B.; Fredel, M.C.; Salmoria, G.V.; et al. Chitosan/β-TCP composites scaffolds coated with silk fibroin: A bone tissue engineering approach. Biomed. Mater. 2022, 17, 015003. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Huang, W.; Xiong, K.; Ruan, S.; Yuan, C.; Mo, G.; Tian, R.; Zhou, S.; She, R.; Ye, P.; et al. Osteochondral repair using scaffolds with gradient pore sizes constructed with silk fibroin, chitosan, and nano-hydroxyapatite. Int. J. Nanomed. 2019, 14, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Pitrolino, K.A.; Felfel, R.M.; Pellizzeri, L.M.; Mlaren, J.; Popov, A.A.; Sottile, V.; Scotchford, C.A.; Scammell, B.E.; Roberts, G.A.F.; Grant, D.M. Development and in vitro assessment of a bi-layered chitosan-nano-hydroxyapatite osteochondral scaffold. Carbohydr. Polym. 2022, 282, 119126. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Moriguchi, Y.; Murawski, C.D.; Yoshikawa, H.; Nakamura, N. Osteochondral tissue engineering with biphasic scaffold: Current strategies and techniques. Tissue Eng. Part B Rev. 2014, 20, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Wang, L.; Liu, Z.; Liu, C. Osteochondral tissue repair in osteoarthritic joints: Clinical challenges and opportunities in tissue engineering. Bio-Des. Manuf. 2018, 1, 101–114. [Google Scholar] [CrossRef]
- Lesage, C.; Lafont, M.; Guihard, P.; Weiss, P.; Guicheux, J.; Delplace, V. Material-Assisted Strategies for Osteochondral Defect Repair. Adv. Sci. 2022, 9, e2200050. [Google Scholar] [CrossRef]
- Browe, D.C.; Díaz-Payno, P.J.; Freeman, F.E.; Schipani, R.; Burdis, R.; Ahern, D.P.; Nulty, J.M.; Guler, S.; Randall, L.D.; Buckley, C.T.; et al. Bilayered extracellular matrix derived scaffolds with anisotropic pore architecture guide tissue organization during osteochondral defect repair. Acta Biomater. 2022, 143, 266–281. [Google Scholar] [CrossRef]
- Zheng, X.; Li, N.; Xu, Y.; Zhang, C.; Ouyang, Y.; Meng, D. A bilayer collagen scaffold with bevacizumab stabilizes chondrogenesis and promotes osteochondral regeneration. Mater. Des. 2022, 221, 110981. [Google Scholar] [CrossRef]
- Silva, S.S.; Rodrigues, L.C.; Fernandes, E.M.; Reis, R.L. Chapter 1—Fundamentals on biopolymers and global demand. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–34. [Google Scholar]
- Islam, N.; Hoque, M.; Taharat, S.F. Recent advances in extraction of chitin and chitosan. World J. Microbiol. Biotechnol. 2022, 39, 28. [Google Scholar] [CrossRef]
- Silva, S.S.; Motta, A.; Rodrigues, M.T.; Pinheiro, A.F.; Gomes, M.E.; Mano, J.F.; Reis, R.L.; Migliaresi, C. Novel genipin-cross-linked chitosan/silk fibroin sponges for cartilage engineering strategies. Biomacromolecules 2008, 9, 2764–2774. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Quan, L.; Ao, Q. Characteristics of Marine Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2022, 20, 372. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Maity, M.; Hasnain, M.S.; Nayak, A.K. Chapter 1—Chitosan: Source, chemistry, and properties. In Chitosan in Drug Delivery; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–22. [Google Scholar]
- Jesús, M.-S.T.; Hernán, H.-M.C.; Rubén, R.-N.J. An overview of the chemical modifications of chitosan and their advantages. Green Mater. 2018, 6, 131–142. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Silva, S.S.; Fernandes, E.M.; Lobo, F.C.M.; Martín-Pastor, M.; Taboada, P.; Reis, R.L. Silk fibroin/cholinium gallate-based architectures as therapeutic tools. Acta Biomater. 2022, 147, 168–184. [Google Scholar] [CrossRef]
- Tuwalska, A.; Grabska-Zielińska, S.; Sionkowska, A. Chitosan/Silk Fibroin Materials for Biomedical Applications—A Review. Polymers 2022, 14, 1343. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Maniglio, D.; Motta, A.; Mano, J.F.; Reis, R.L.; Migliaresi, C. Genipin-modified silk-fibroin nanometric nets. Macromol. Biosci. 2008, 8, 766–774. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Yoshida, M.; Turner, P.R.; McAdam, C.J.; Ali, M.A.; Cabral, J.D. A comparison between β-tricalcium phosphate and chitosan poly-caprolactone-based 3D melt extruded composite scaffolds. Biopolymers 2022, 113, e23482. [Google Scholar] [CrossRef]
- Ahlhelm, M.; Latorre, S.H.; Mayr, H.O.; Storch, C.; Freytag, C.; Werner, D.; Schwarzer-Fischer, E.; Seidenstücker, M. Mechanically Stable β-TCP Structural Hybrid Scaffolds for Potential Bone Replacement. J. Compos. Sci. 2021, 5, 281. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Ribeiro, N.; Nunes, C.M.M.; Rodrigues, A.F.M.; Sousa, A.; Olhero, S.M. Toughening robocast chitosan/biphasic calcium phosphate composite scaffolds with silk fibroin: Tuning printable inks and scaffold structure for bone regeneration. Biomater. Adv. 2022, 134, 112690. [Google Scholar] [CrossRef]
- Shi, W.; Liu, D.; Tan, C.; Sun, Z.; Bai, P.; Yin, J.; Mao, C.; Lin, J.; Yang, H. Preparation of Calcium Phosphate Composite Cement Incorporated with Silk Fibroin and Chitosan-N-Acetylcysteine Nanoparticles and In Vitro Bioactivities Analysis. J. Biomater. Tissue Eng. 2018, 8, 1356–1363. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, M.; Xu, B.; Zhang, J.; Zhao, Y.; Ji, S.; Wang, L.; Wang, L.; Li, X.; Kong, D.; et al. Integrated Trilayered Silk Fibroin Scaffold for Osteochondral Differentiation of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2014, 6, 16696–16705. [Google Scholar] [CrossRef] [PubMed]
- Signini, R.; Filho, S. Characteristics and properties of purified chitosan in the neutral, acetate and hydrochloride forms. Polímeros 2001, 11, 58–64. [Google Scholar] [CrossRef]
- Jinlong, N.; Zhenxi, Z.; Dazong, J. Investigation of Phase Evolution During the Thermochemical Synthesis of Tricalcium Phosphate. J. Mater. Synth. Process. 2001, 9, 235–240. [Google Scholar] [CrossRef]
- Kim, U.-J.; Park, J.; Joo Kim, H.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.C.; Yang, D.; Ryser, H.J. Colorimetric determination of microgram quantities of polylysine by trypan blue precipitation. Anal. Biochem. 1984, 142, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, X.; Pan, L.; Deng, Y.; Zheng, H.; Peng, Z.; Wan, J.; Zhou, Y.; Wang, B. Molecular Evidence of Structural Changes in Silk Using Unlimited Degradation Mass Spectrometry. ACS Omega 2023, 8, 34410–34419. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; Reis, R.L.; Cunha, A.M.; Blitterswijk, C.A.; de Bruijn, J.D. Cytocompatibility and response of osteoblastic-like cells to starch-based polymers: Effect of several additives and processing conditions. Biomaterials 2001, 22, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: London, UK, 2006. [Google Scholar]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 11, 2041731420974861. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Sung, H.-W.; Shyu, S.-S. Synthesis and characterization of a novel chitosan-based network prepared using naturally occurring crosslinker. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2804–2814. [Google Scholar] [CrossRef]
- Reay, S.L.; Jackson, E.L.; Ferreira, A.M.; Hilkens, C.M.U.; Novakovic, K. In vitro evaluation of the biodegradability of chitosan–genipin hydrogels. Mater. Adv. 2022, 3, 7946–7959. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Guo, T. Genipin-crosslinked hydrophobical chitosan microspheres and their interactions with bovine serum albumin. Carbohydr. Polym. 2011, 83, 2016–2021. [Google Scholar] [CrossRef]
- Grzybek, P.; Jakubski, Ł.; Dudek, G. Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application. Int. J. Mol. Sci. 2022, 23, 9932. [Google Scholar] [CrossRef]
- Heimbuck, A.M.; Priddy-Arrington, T.R.; Sawyer, B.J.; Caldorera-Moore, M.E. Effects of post-processing methods on chitosan-genipin hydrogel properties. Mater. Sci. Eng. C 2019, 98, 612–618. [Google Scholar] [CrossRef]
- Yong, Z.; Ming, N.; Miqin, Z.; Buddy, R. Calcium Phosphate—Chitosan Composite Scaffolds for Bone Tissue Engineering. J. Tissue Eng. 2004, 9, 337–345. [Google Scholar]
- Sherwood, J.K.; Riley, S.L.; Palazzolo, R.; Brown, S.C.; Monkhouse, D.C.; Coates, M.; Griffith, L.G.; Landeen, L.K.; Ratcliffe, A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 2002, 23, 4739–4751. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.-L.; Dai, F.-Y.; Zhang, H.-H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y.-Z. Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery. J. Transl. Med. 2012, 10, 117. [Google Scholar] [CrossRef]
- Siddiqui, N.; Pramanik, K.; Jabbari, E. Osteogenic differentiation of human mesenchymal stem cells in freeze-gelled chitosan/nano β-tricalcium phosphate porous scaffolds crosslinked with genipin. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Moreira, C.D.F.; Costa-Júnior, E.S.; Pereira, M.M. In vitro degradation of chitosan composite foams for biomedical applications and effect of bioactive glass as a crosslinker. Biomed. Glas. 2018, 4, 45–56. [Google Scholar] [CrossRef]
- Lowe, B.; Ottensmeyer, M.P.; Xu, C.; He, Y.; Ye, Q.; Troulis, M.J. The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective. J. Funct. Biomater. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.S.; Lee, W.F.; Lin, P.Y.; Wu, Y.-F.; Huang, H.-M.; Teng, N.-C.; Pan, Y.-H.; Salamanca, E.; Chang, W.-J. Surface Modified β-Tricalcium phosphate enhanced stem cell osteogenic differentiation in vitro and bone regeneration in vivo. Sci. Rep. 2021, 11, 9234. [Google Scholar] [CrossRef] [PubMed]
- Knabe, C.; Ducheyne, P. Bioactivity: Mechanisms. Compr. Biomater. 2011, 1, 245–258. [Google Scholar] [CrossRef]
- Massit, A.; Chafik El Idrissi, B.; Yamni, K. Synthesis and characterization of nano-sized β-Tricalcium phosphate: Effects of the aging time. IOSR J. Appl. Chem. 2014, 7, 57–61. [Google Scholar]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Ching, K.Y.; Andriotis, O.; Sengers, B.; Stolz, M. Genipin crosslinked chitosan/PEO nanofibrous scaffolds exhibiting an improved microenvironment for the regeneration of articular cartilage. J. Biomater. Appl. 2021, 36, 503–516. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Andrea, L.; Marica, I.; Anamarija, R. Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds. J. Tissue Repair Regen. 2017, 1, 12–22. [Google Scholar] [CrossRef]
- Qasim, S.B.; Husain, S.; Huang, Y.; Pogorielov, M.; Deineka, V.; Lyndin, M.; Rawlinson, A.; Rehman, I.U. In-vitro and in-vivo degradation studies of freeze gelated porous chitosan composite scaffolds for tissue engineering applications. Polym. Degrad. Stab. 2017, 136, 31–38. [Google Scholar] [CrossRef]
- Pighinelli, L.; Kucharska, M.; Wísniewska-Wrona, M.; Gruchała, B.; Brzoza-Malczewska, K. Biodegradation study of microcrystalline chitosan and microcrystalline chitosan/β-TCP complex composites. Int. J. Mol. Sci. 2012, 13, 7617–7628. [Google Scholar] [CrossRef]
- Roffi, A.; Kon, E.; Perdisa, F.; Fini, M.; Di Martino, A.; Parrilli, A.; Salamanna, F.; Sandri, M.; Sartori, M.; Sprio, S.; et al. A Composite Chitosan-Reinforced Scaffold Fails to Provide Osteochondral Regeneration. Int. J. Mol. Sci. 2019, 20, 2227. [Google Scholar] [CrossRef]
- Srinivasan, S.; Kumar, P.; Chennazhi, K.; Nair, S.; Tamura, H.; Jayakumar, R. Biocompatible β-chitin Hydrogel/Nanobioactive Glass Ceramic Nanocomposite Scaffolds for Periodontal Bone Regeneration. Trends Biomater. Artif. Organs 2011, 25, 1–11. [Google Scholar]
- Cheng, G.; Davoudi, Z.; Xing, X.; Yu, X.; Cheng, X.; Li, Z.; Deng, H.; Wang, Q. Advanced Silk Fibroin Biomaterials for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 2704–2715. [Google Scholar] [CrossRef]
- Gokila, S.; Gomathi, T.; Kumar, V.; Alshahrani, F.; Anil, S.; Sudha, P.N. Development of 3D scaffolds using nanochitosan/silk-fibroin/hyaluronic acid biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2018, 120, 876–885. [Google Scholar] [CrossRef]
- Doblaré, M.; García, J.M.; Gómez, M.J. Modelling bone tissue fracture and healing: A review. Eng. Fract. Mech. 2004, 71, 1809–1840. [Google Scholar] [CrossRef]
- Sasaki, T.; Amizuka, N.; Irie, K.; Ejiri, S.; Ozawa, H. Localization of Alkaline Phosphatase and Osteopontin during Matrix Mineralization in the Developing Cartilage of Coccygeal Vertebrae. Arch. Histol. Cytol. 2000, 63, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Canadas, R.F.; Jiménez, G.; Perán, M.; Marchal, J.A.; Reis, R.L.; Oliveira, J.M. Biofunctional Ionic-Doped Calcium Phosphates: Silk Fibroin Composites for Bone Tissue Engineering Scaffolding. Cells Tissues Organs 2017, 204, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Amann, E.; Amirall, A.; Franco, A.R.; Poh, P.S.P.; Sola Dueñas, F.J.; Fuentes Estévez, G.; Leonor, I.B.; Reis, R.L.; van Griensven, M.; Balmayor, E.R. A Graded, Porous Composite of Natural Biopolymers and Octacalcium Phosphate Guides Osteochondral Differentiation of Stem Cells. Adv. Healthc. Mater. 2021, 10, 2001692. [Google Scholar] [CrossRef] [PubMed]










| Sample | Bone Layer | Cartilage Layer | |
|---|---|---|---|
| %CHT (w/v) | % β-TCP (w/v) | % SF (w/v) | |
| CSG | 3 | - | 4.2 |
| CBSG10 | 3 | 10 | 4.2 |
| CBSG20 | 3 | 20 | 4.2 |
| Samples | Area | Mean Thickness (µm) | Mean Pore Size (µm) | Porosity (%) |
|---|---|---|---|---|
| CSG | Top | 35.91 ± 4.14 | 73.71 ± 11.22 | 62.91 ± 3.52 |
| Interface | 44.58 ± 2.02 | 65.22 ± 5.44 | 62.40 ± 2.09 | |
| Bottom | 42.12 ± 5.72 | 77.37 ± 1.15 | 69.83 ± 1.73 | |
| CBSG10 | Top | 42.22 ± 16.71 | 84.12 ± 23.33 | 71.21 ± 9.77 |
| Interface | 80.27 ± 3.75 | 47.10 ± 2.79 | 31.55 ± 0.64 | |
| Bottom | 100.17 ± 15.24 | 35.73 ± 4.04 | 18.15 ± 7.82 | |
| CBSG20 | Top | 44.18 ± 8.79 | 58.46 ± 2.95 | 54.42 ± 0.68 |
| Interface | 57.94 ± 9.14 | 30.91 ± 3.13 | 31.20 ± 1.06 | |
| Bottom | 103.86 ± 15.06 | 25.85 ± 2.32 | 8.71 ± 5.74 |
| Sample | Elastic Modulus (MPa) | Yield Strength (KPa) |
|---|---|---|
| CSG | 3.0 ± 1.5 | 118.7 ± 16.6 |
| CBSG10 | 3.3 ± 1.5 | 160.5 ± 51.5 |
| CBSG20 | 1.1 ± 0.7 | 106.1 ± 57.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piaia, L.; Silva, S.S.; Fernandes, E.M.; Gomes, J.M.; Franco, A.R.; Leonor, I.B.; Fredel, M.C.; Salmoria, G.V.; Hotza, D.; Reis, R.L. Chitosan-Based Hierarchical Scaffolds Crosslinked with Genipin. J. Compos. Sci. 2024, 8, 85. https://doi.org/10.3390/jcs8030085
Piaia L, Silva SS, Fernandes EM, Gomes JM, Franco AR, Leonor IB, Fredel MC, Salmoria GV, Hotza D, Reis RL. Chitosan-Based Hierarchical Scaffolds Crosslinked with Genipin. Journal of Composites Science. 2024; 8(3):85. https://doi.org/10.3390/jcs8030085
Chicago/Turabian StylePiaia, Lya, Simone S. Silva, Emanuel M. Fernandes, Joana M. Gomes, Albina R. Franco, Isabel B. Leonor, Márcio C. Fredel, Gean V. Salmoria, Dachamir Hotza, and Rui L. Reis. 2024. "Chitosan-Based Hierarchical Scaffolds Crosslinked with Genipin" Journal of Composites Science 8, no. 3: 85. https://doi.org/10.3390/jcs8030085
APA StylePiaia, L., Silva, S. S., Fernandes, E. M., Gomes, J. M., Franco, A. R., Leonor, I. B., Fredel, M. C., Salmoria, G. V., Hotza, D., & Reis, R. L. (2024). Chitosan-Based Hierarchical Scaffolds Crosslinked with Genipin. Journal of Composites Science, 8(3), 85. https://doi.org/10.3390/jcs8030085