The Effect of Substrate Temperature on the Evaporative Behaviour and Desiccation Patterns of Foetal Bovine Serum Drops
Abstract
:1. Introduction
2. Materials and Methods
3. Results
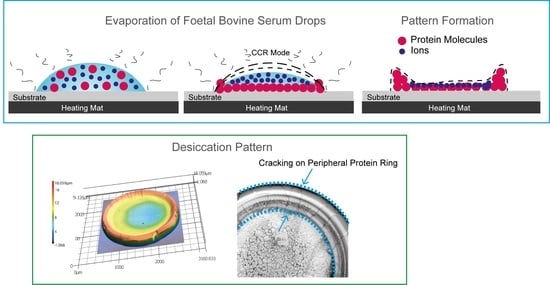
3.1. Evaporation Stages
3.1.1. Pre-Gelation
3.1.2. Gelation
3.1.3. Crystallisation and Crack Formation
3.2. The Effect of Temperature on the Morphology and Topography of the Final Desiccation Deposits
4. Discussion
Glassy Peripheral Protein Ring and Crack Formation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Xu, X.; Luo, J. Marangoni flow in an evaporating water droplet. Appl. Phys. Lett. 2007, 91, 124102. [Google Scholar] [CrossRef]
- Semenov, S.; Starov, V.M.; Rubio, R.G.; Agogo, H.; Velarde, M.G. Evaporation of sessile water droplets: Universal behaviour in presence of contact angle hysteresis. Colloids Surf. A Physicochem. Eng. Asp. 2011, 391, 135–144. [Google Scholar] [CrossRef]
- Misyura, S.Y. Evaporation of a sessile water drop and a drop of aqueous salt solution. Sci. Rep. 2017, 7, 14759. [Google Scholar] [CrossRef] [Green Version]
- Christy, J.R.E.; Hamamoto, Y.; Sefiane, K. Flow Transition within an Evaporating Binary Mixture Sessile Drop. Phys. Rev. Lett. 2011, 106, 205701. [Google Scholar] [CrossRef] [PubMed]
- Sefiane, K.; David, S.; Shanahan, M.E.R. Wetting and evaporation of binary mixture drops. J. Phys. Chem. B 2008, 112, 11317–11323. [Google Scholar] [CrossRef]
- Liu, W.; Midya, J.; Kappl, M.; Butt, H.J.; Nikoubashman, A. Segregation in Drying Binary Colloidal Droplets. ACS Nano 2019, 13, 4972–4979. [Google Scholar] [CrossRef]
- Maki, K.L.; Kumar, S. Fast evaporation of spreading droplets of colloidal suspensions. Langmuir 2011, 27, 11347–11363. [Google Scholar] [CrossRef]
- Patil, N.D.; Bange, P.G.; Bhardwaj, R.; Sharma, A. Effects of Substrate Heating and Wettability on Evaporation Dynamics and Deposition Patterns for a Sessile Water Droplet Containing Colloidal Particles. Langmuir 2016, 32, 11958–11972. [Google Scholar] [CrossRef] [Green Version]
- Sung, P.F.; Wang, L.; Harris, M.T. Deposition of Colloidal Particles during the Evaporation of Sessile Drops: Dilute Colloidal Dispersions. Int. J. Chem. Eng. 2019, 2019, 7954965. [Google Scholar] [CrossRef]
- Choudhury, M.D.; Dutta, T.; Tarafdar, S. Pattern formation in droplets of starch gels containing NaCl dried on different surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2013, 432, 110–118. [Google Scholar] [CrossRef]
- Kaya, D.; Belyi, V.A.; Muthukumar, M. Pattern formation in drying droplets of polyelectrolyte and salt. J. Chem. Phys. 2010, 133, 114905. [Google Scholar] [CrossRef]
- Takhistov, P.; Chang, H. Complex Stain Morphologies. Ind. Eng. Chem. Res. 2002, 41, 6256–6269. [Google Scholar] [CrossRef]
- Yakhno, T. Salt-induced protein phase transitions in drying drops. J. Colloid Interface Sci. 2008, 318, 225–230. [Google Scholar] [CrossRef]
- Sobac, B.; Brutin, D. Desiccation of a sessile drop of blood: Cracks, folds formation and delamination. Colloids Surf. A Physicochem. Eng. Asp. 2014, 448, 34–44. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Zang, D.; Shen, W. Blood drop patterns: Formation and applications. Adv. Colloid Interface Sci. 2016, 231, 1–14. [Google Scholar] [CrossRef]
- Annarelli, C.C.; Fornazero, J.; Bert, J.; Colombani, J. Crack Patterns in drying protein solution drops. Eur. Phys. J. E 2001, 603, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Gorr, H.M.; Zueger, J.M.; McAdams, D.R.; Barnard, J.A. Salt-induced pattern formation in evaporating droplets of lysozyme solutions. Colloids Surf. B Biointerfaces 2013, 103, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Pauchard, L.; Parisse, F.; Allain, C. Influence of salt content on crack patterns formed through colloidal suspension desiccation. Phys. Rev. E 1999, 59, 3737–3740. [Google Scholar] [CrossRef]
- Tarasevich, Y.Y.; Ayupova, A.K. Effect of diffusion on the separation of components in a biological fluid upon wedge-shaped dehydration. Tech. Phys. 2003, 48, 535–540. [Google Scholar] [CrossRef]
- Zhong, X.; Ren, J.; Duan, F. Wettability Effect on Evaporation Dynamics and Crystalline Patterns of Sessile Saline Droplets. J. Phys. Chem. B 2017, 121, 7924–7933. [Google Scholar] [CrossRef] [PubMed]
- Soulié, V.; Karpitschka, S.; Lequien, F.; Prené, P.; Zemb, T.; Moehwald, H.; Riegler, H. The evaporation behavior of sessile droplets from aqueous saline solutions. Phys. Chem. Chem. Phys. 2015, 17, 22296–22303. [Google Scholar] [CrossRef] [PubMed]
- Shahidzadeh, N.; Schut, M.F.L.; Desarnaud, J.; Prat, M.; Bonn, D. Salt stains from evaporating droplets. Sci. Rep. 2015, 5, 10335. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Siedlecki, C.A.; Vogler, E.A. Mixology of protein solutions and the Vroman effect. Langmuir 2004, 20, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Shahidzadeh-Bonn, N.; Rafai, S.; Bonn, D.; Wegdam, G. Salt crystallization during evaporation: Impact of interfacial properties. Langmuir 2008, 24, 8599–8605. [Google Scholar] [CrossRef]
- Killeen, A.A.; Ossina, N.; Mcglennen, R.C.; Minnerath, S.; Borgos, J. Protein Self-Organization Patterns in Dried Serum Reveal Changes in B-Cell Disorders. Mol. Diagn. Ther. 2006, 10, 371–380. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Analysis of the Effects of Marangoni Stresses on the Microflow in an Evaporating Sessile Droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef]
- Han, W.; Lin, Z. Learning from “coffee rings”: Ordered structures enabled by controlled evaporative self-assembly. Angew. Chem. Int. Ed. 2012, 51, 1534–1546. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, S.; Guo, C.; He, M.; Chen, D.; Ji, Y.; Chen, Z.; Wu, X.; Liu, Q.; Xie, S. Controllable two-stage droplet evaporation method and its nanoparticle self-assembly mechanism. Langmuir 2013, 29, 6232–6241. [Google Scholar] [CrossRef]
- Laan, N.; De Bruin, K.G.; Slenter, D.; Wilhelm, J.; Jermy, M.; Bonn, D. Bloodstain Pattern Analysis: Implementation of a fluid dynamic model for position determination of victims. Sci. Rep. 2015, 5, 11461. [Google Scholar] [CrossRef] [PubMed]
- Brutin, D.; Sobac, B.; Loquet, B.; Sampol, J. Pattern formation in drying drops of blood. J. Fluid Mech. 2011, 667, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Yakhno, T.A.; Yakhno, V.G.; Sanin, A.G.; Sanina, O.A.; Pelyushenko, A.S.; Egorova, N.A.; Terentiev, I.G.; Smetanina, S.V.; Korochkina, O.V.; Yashukova, E.V. The informative-capacity phenomenon of drying drops. IEEE Eng. Med. Biol. Mag. 2005, 24, 96–104. [Google Scholar] [CrossRef]
- Smith, F.R.; Nicloux, C.; Brutin, D. A new forensic tool to date human blood pools. Sci. Rep. 2020, 10, 8598. [Google Scholar] [CrossRef]
- Yakhno, T.A.; Yakhno, V.G. Structural evolution of drying drops of biological fluids. Tech. Phys. 2009, 54, 1219–1227. [Google Scholar] [CrossRef]
- Shabalin, V.N.; Shatokhina, S.N. Diagnostic markers in the structures of human biological liquids. Singapore Med. J. 2007, 48, 440–446. [Google Scholar]
- Yakhno, T.A.; Sedova, O.A.; Sanin, A.G.; Pelyushenko, A.S. On the Existence of Regular Structures in Liquid Human Blood Serum (Plasma) and Phase Transitions in the Course of Its Drying. Tech. Phys. 2003, 48, 399–403. [Google Scholar] [CrossRef]
- Roy, B.; Choudhuri, M.D.; Dutta, T.; Tarafdar, S. Multi-scale patterns formed by sodium sulphate in a drying droplet of gelatin. Appl. Surf. Sci. 2015, 357, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Gope, A.; Athair, A.S.; Iannacchione, G.S. A comparative study of the drying evolution and dried morphology of two globular proteins in de-ionized water solutions. RSC Adv. 2020, 10, 16906–16916. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Iannacchione, G.S. Image-Based Analysis of Patterns Formed in Drying Drops. In Proceedings of the International Conference on Pattern Recognition and Machine Intelligence 2019, Tezpur, India, 17–20 December 2019; pp. 567–574. [Google Scholar] [CrossRef] [Green Version]
- Annarelli, C.C.; Reyes, L.; Fornazero, J.; Bert, J. Ion and molecular recognition effects on the crystallisation of bovine serum albumin—Salt mixtures. Cryst. Eng. 2000, 3, 173–194. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; He, H.; Shen, W. Desiccation Patterns of Plasma Sessile Drops. ACS Sens. 2019, 4, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Esmonde-white, K.A.; Esmonde-white, F.W.L.; Morris, M.D.; Blake, J.; Arbor, A.; Arbor, A. Characterization of Biofluids Prepared by Sessile Drop Formation. Analyst 2015, 139, 2734–2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brutin, D.; Sobac, B. Influence of Substrate Nature on the Evaporation of a Sessile Drop of Blood. J. Heat Trasnfer 2012, 134, 061101. [Google Scholar] [CrossRef]
- Zeid, W.B.; Brutin, D. Influence of relative humidity on spreading, pattern formation and adhesion of a drying drop of whole blood. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 1–7. [Google Scholar] [CrossRef]
- Iqbal, R.; Shen, A.Q.; Sen, A.K. Understanding of the role of dilution on evaporative deposition patterns of blood droplets over hydrophilic and hydrophobic substrates. J. Colloid Interface Sci. 2020, 579, 541–550. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Iannacchione, G. Temperature and concentration dependence of human whole blood and protein drying droplets. Biomolecules 2021, 11, 231. [Google Scholar] [CrossRef]
- Carreón, Y.J.P.; Ríos-Ramírez, M.; Vázquez-Vergara, P.; Salinas-Almaguer, S.; Cipriano-Urbano, I.; Briones-Aranda, A.; Díaz-Hernández, O.; Escalera Santos, G.J.; González-Gutiérrez, J. Effects of substrate temperature on patterns produced by dried droplets of proteins. Colloids Surf. B Biointerfaces 2021, 203, 111763. [Google Scholar] [CrossRef]
- Shahidzadeh-Bonn, N.; Rafaï, S.; Azouni, A.; Bonn, D. Evaporating droplets. J. Fluid Mech. 2006, 549, 307–313. [Google Scholar] [CrossRef]
- Carrier, O.; Shahidzadeh-Bonn, N.; Zargar, R.; Aytouna, M.; Habibi, M.; Eggers, J.; Bonn, D. Evaporation of water: Evaporation rate and collective effects. J. Fluid Mech. 2016, 798, 774–786. [Google Scholar] [CrossRef] [Green Version]
- Birdi, K.S.; Vu, D.T. Wettability and the evaporation rates of fluids from solid surfaces. J. Adhes. Sci. Technol. 1993, 7, 485–493. [Google Scholar] [CrossRef]
- Picknett, R.G.; Bexon, R. The evaporation of sessile or pendant drops in still air. J. Colloid Interface Sci. 1977, 61, 336–350. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Zang, D.; Shen, W. Understanding desiccation patterns of blood sessile drops. J. Mater. Chem. B 2017, 5, 8991–8998. [Google Scholar] [CrossRef] [PubMed]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Bhomia, R.; Trivedi, V.; Coleman, N.J.; Mitchell, J.C. The thermal and storage stability of bovine haemoglobin by ultraviolet–visible and circular dichroism spectroscopies. J. Pharm. Anal. 2016, 6, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Digel, I.; Maggakis-Kelemen, C.; Zerlin, K.F.; Linder, P.; Kasischke, N.; Kayser, P.; Porst, D.; Artmann, A.T.; Artmann, G.M. Body temperature-related structural transitions of monotremal and human hemoglobin. Biophys. J. 2006, 91, 3014–3021. [Google Scholar] [CrossRef] [Green Version]
- Artmann, G.M.; Burns, L.; Canaves, J.M.; Temiz-Artmann, A.; Schmid-Schönbein, G.W.; Chien, S.; Maggakis-Kelemen, C. Circular dichroism spectra of human hemoglobin reveal a reversible structural transition at body temperature. Eur. Biophys. J. 2004, 33, 490–496. [Google Scholar] [CrossRef]
- Yakhno, T.A.; Yakhno, V.G.; Sanin, A.G.; Sanina, O.A.; Pelyushenko, A.S. Protein and salt: Spatiotemporal dynamics of events in a drying drop. Tech. Phys. 2004, 49, 1055–1063. [Google Scholar] [CrossRef]
- Li, Y.; Lv, C.; Li, Z.; Quéré, D.; Zheng, Q. From coffee rings to coffee eyes. Soft Matter 2015, 11, 4669–4673. [Google Scholar] [CrossRef]
- Lama, H.; Basavaraj, M.G.; Satapathy, D.K. Tailoring crack morphology in coffee-ring deposits: Via substrate heating. Soft Matter 2017, 13, 5445–5452. [Google Scholar] [CrossRef]
- Griffith, A.A. The phenomena of rupture and flow in solids. Philos. Trans. R. Soc. Lond. Ser. A 1921, 221, 163–198. [Google Scholar] [CrossRef] [Green Version]
- Goehring, L.; Nakahara, A.; Dutta, T.; Kitsunezaki, S.; Tarafdar, S. Desiccation Cracks and Their Patterns; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; ISBN 9783527671922. [Google Scholar]
- Bonn, D.; Kellay, H.; Prochnow, M.; Ben-Djemiaa, K.; Meunier, J. Delayed fracture of an inhomogeneous soft solid. Science 1998, 280, 265–267. [Google Scholar] [CrossRef]














| Type of Protein | Typical Concentration Range | Concentration in Our Sample |
|---|---|---|
| BSA | 17–35 mg/mL | 23 mg/mL |
| α-globulin | 7–20 mg/mL | 16 mg/mL |
| β-globulin | 3–9 mg/mL | 3.6 mg/mL |
| γ-globulin | 10–200 μg/mL | 23.73 μg/mL |
| Haemoglobin | 0.01–0.30 mg/mL | 0.1401 mg/mL |
| Temperature (°C) | Saturated Vapour Pressure of Water |
|---|---|
| 20 | 2.33 |
| 25 | 3.17 |
| 30 | 4.24 |
| 35 | 5.63 |
| 40 | 7.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efstratiou, M.; Christy, J.; Bonn, D.; Sefiane, K. The Effect of Substrate Temperature on the Evaporative Behaviour and Desiccation Patterns of Foetal Bovine Serum Drops. Colloids Interfaces 2021, 5, 43. https://doi.org/10.3390/colloids5040043
Efstratiou M, Christy J, Bonn D, Sefiane K. The Effect of Substrate Temperature on the Evaporative Behaviour and Desiccation Patterns of Foetal Bovine Serum Drops. Colloids and Interfaces. 2021; 5(4):43. https://doi.org/10.3390/colloids5040043
Chicago/Turabian StyleEfstratiou, Marina, John Christy, Daniel Bonn, and Khellil Sefiane. 2021. "The Effect of Substrate Temperature on the Evaporative Behaviour and Desiccation Patterns of Foetal Bovine Serum Drops" Colloids and Interfaces 5, no. 4: 43. https://doi.org/10.3390/colloids5040043
APA StyleEfstratiou, M., Christy, J., Bonn, D., & Sefiane, K. (2021). The Effect of Substrate Temperature on the Evaporative Behaviour and Desiccation Patterns of Foetal Bovine Serum Drops. Colloids and Interfaces, 5(4), 43. https://doi.org/10.3390/colloids5040043