The Relevance of Astrocytic Cell Culture Models for Neuroinflammation in Neurodegeneration Research
Abstract
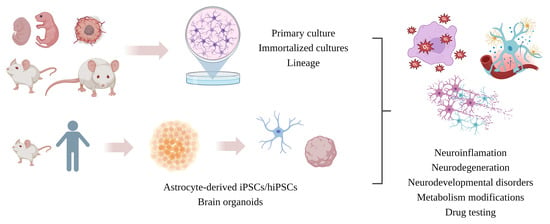
:1. Introduction
2. A Brief Overview of Astrocytes General Features and Physiological Roles
3. Reactive Astrocytes and Pathological States
4. Experimental Readouts for Reactive Astrocytes in Inflammation Context
5. Astrocytic In Vitro Models
5.1. Primary Cell Culture
5.2. Cell Lines
5.3. Immortalized Astrocytes (IAs)
5.4. iPSC-Derived Astrocytes
5.5. 3D Culture and Bioprinting
5.6. Neurospheres and Brain Organoids (Mini-Brain)
6. Major Differences between Cell Cultures: Implications for Neuroinflammation Associated with Neurodegeneration
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valentin, G. Über Den Verlauf und Die Letzten Enden Der Nerven; Gedruckt bei Grass, Barth und Comp.: Breslau, Poland, 1836; Volume 18. [Google Scholar]
- Deiters, O.; Schultze, M.J.S. Untersuchungen Über Gehirn und Rückenmark Des Menschen und Der Säugethiere; F. Veiweg: Braunschweig, Germany, 1865. [Google Scholar]
- Gesammelte Abhandlungen zur Wissenschaftlichen Medicin/Von Rudolf Virchow. Available online: https://wellcomecollection.org/works/m3tp5x6w (accessed on 28 December 2023).
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 42, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Lyons, D.A. Glia as Architects of Central Nervous System Formation and Function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- von Lenhossék, M. Zur Kenntnis der Neuroglia des menschlichen Rückenmarkes. Verh. Anat. Ges. 1891, 5, 193–221. [Google Scholar]
- Robertson, W. On a New Method of Obtaining a Black Reaction in Certain Tissue-Elements of the Central Nervous System. Scott. Med. Surg. J. 1899, 4, 23. [Google Scholar]
- Río-Hortega, P. del Son Homologables La Glía de Escasas Radiaciones y La Célula de Schwann. Boletıín Soc. Esp. Biol. 1922, 10, 25–28. [Google Scholar]
- Penfield, W. Oligodendroglia and Its Relation to Classical Neuroglia. Brain 1924, 47, 430–452. [Google Scholar] [CrossRef]
- Geren, B.B. The Formation from the Schwann Cell Surface of Myelin in the Peripheral Nerves of Chick Embryos. Exp. Cell Res. 1954, 7, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Bunge, M.B.; Bunge, R.P.; Pappas, G.D. Electron Microscopic Demonstration of Connections between Glia and Myelin Sheaths in the Developing Mammalian Central Nervous System. J. Cell Biol. 1962, 12, 448–453. [Google Scholar] [CrossRef]
- Río-Hortega, P.D.; Penfield, W. Cerebral Cicatrix—The Reaction of Neuroglia and Microglia to Brain Wounds. Johns Hopkins Hosp. Bull. 1927, 41, 278–303. [Google Scholar]
- Lugaro, E. Sulle Funzioni Della Nevroglia. Riv. Pat. Nerv. Ment. 1907, 12, 225–233. [Google Scholar]
- Nageotte, J. Pheénomenès de Sécrétion Dans Le Protoplasma Des Cellules Neévrogliques de La Substance Grise. CR Soc. Biol. 1910, 68, 1068–1069. [Google Scholar]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.; Jeftinija, S.; Haydon, P.G. Glutamate-Mediated Astrocyte-Neuron Signalling. Nature 1994, 369, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite Synapses: Glia, the Unacknowledged Partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cajal, S.R. Algunas Conjeturas Sobre El Mecanismo Anatómico de La Ideación, Asociación y Atención. Rev. Med. Cirug. Pract. 1895, 36, 497–508. [Google Scholar]
- Cajal, S.R. Algo Sobre La Significacion Fisiologica de La Consejos Acerca de La Tecnica Del Oro-Sublimado. Trab. Lab. Investig. Biol. Neurogl. Rev. Trim. Microg. 1897, 33–47. [Google Scholar]
- Cajal, S.R. El Encéfalo de Los Reptiles. Trab. Lab. Histol. Fac. Zarag. 1891, 24, 1–31. [Google Scholar]
- Parpura, V.; Verkhratsky, A. Neuroglia at the Crossroads of Homoeostasis, Metabolism and Signalling: Evolution of the Concept. ASN Neuro 2012, 4, e00087. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate Immune Activation in Neurodegenerative Disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 1–32. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Golgi, C. Sulla Fina Struttura Dei Bulbi Olfattorii; Tipografia di Stefano Calderini: Reggio nell’Emilia, Italy, 1875. [Google Scholar]
- HIs, W. Zur Geschichte des Gehirns Sowie der Centralen und Peripherischen Nervenbahnen Beim Menschlichen Embryo; Königlich Sächsische Gesellschaft der Wissenschaften: Leipzig, Germany, 1888; Volume 24, pp. 341–392. [Google Scholar]
- Cajal, S.R. La Rétine Des Vertébrés. La Cellule 1893, 9, 119–257. [Google Scholar]
- Tessier-Lavigne, M.; Goodman, C.S. The Molecular Biology of Axon Guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Bentivoglio, M.; Mazzarello, P. The History of Radial Glia. Brain Res. Bull. 1999, 49, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, C. The Chemotactic Hypothesis of Cajal: A Century Behind. Prog. Brain Res. 2002, 136, 11–20. [Google Scholar] [CrossRef]
- Hösli, L.; Zuend, M.; Bredell, G.; Zanker, H.S.; Oliveira, C.E.P.D.; Saab, A.S.; Weber, B. Direct Vascular Contact Is a Hallmark of Cerebral Astrocytes. Cell Rep. 2022, 39. [Google Scholar] [CrossRef]
- Bartheld, C.S.V.; Bahney, J.; Herculano-Houzel, S. The Search for True Numbers of Neurons and Glial Cells in the Human Brain: A Review of 150 Years of Cell Counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef]
- Bahney, J.; von Bartheld, C.S. The Cellular Composition and Glia–Neuron Ratio in the Spinal Cord of a Human and a Nonhuman Primate: Comparison With Other Species and Brain Regions. Anat. Rec. 2018, 301, 697–710. [Google Scholar] [CrossRef]
- Sherwood, C.C.; Stimpson, C.D.; Raghanti, M.A.; Wildman, D.E.; Uddin, M.; Grossman, L.I.; Goodman, M.; Redmond, J.C.; Bonar, C.J.; Erwin, J.M.; et al. Evolution of Increased Glia–Neuron Ratios in the Human Frontal Cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 13606–13611. [Google Scholar] [CrossRef]
- Ruiz-Sauri, A.; Orduña-Valls, J.M.; Blasco-Serra, A.; Tornero-Tornero, C.; Cedeño, D.L.; Bejarano-Quisoboni, D.; Valverde-Navarro, A.A.; Benyamin, R.; Vallejo, R. Glia to Neuron Ratio in the Posterior Aspect of the Human Spinal Cord at Thoracic Segments Relevant to Spinal Cord Stimulation. J. Anat. 2019, 235, 997–1006. [Google Scholar] [CrossRef]
- Lovick, T.A.; Brown, L.A.; Key, B.J. Neuronal Activity-Related Coupling in Cortical Arterioles: Involvement of Astrocyte-Derived Factors. Exp. Physiol. 2005, 90, 131–140. [Google Scholar] [CrossRef]
- Shigetomi, E.; Kracun, S.; Sofroniew, M.V.; Khakh, B.S. A Genetically Targeted Optical Sensor to Monitor Calcium Signals in Astrocyte Processes. Nat. Neurosci. 2010, 13, 759–766. [Google Scholar] [CrossRef]
- Di Castro, M.A.; Chuquet, J.; Liaudet, N.; Bhaukaurally, K.; Santello, M.; Bouvier, D.; Tiret, P.; Volterra, A. Local Ca2+ Detection and Modulation of Synaptic Release by Astrocytes. Nat. Neurosci. 2011, 14, 1276–1284. [Google Scholar] [CrossRef]
- Nett, W.J.; Oloff, S.H.; McCarthy, K.D. Hippocampal Astrocytes in Situ Exhibit Calcium Oscillations That Occur Independent of Neuronal Activity. J. Neurophysiol. 2002, 87, 528–537. [Google Scholar] [CrossRef]
- De Bock, M.; Decrock, E.; Wang, N.; Bol, M.; Vinken, M.; Bultynck, G.; Leybaert, L. The Dual Face of Connexin-Based Astroglial Ca2+ Communication: A Key Player in Brain Physiology and a Prime Target in Pathology. Biochim. Biophys. Acta 2014, 1843, 2211–2232. [Google Scholar] [CrossRef]
- Allen, N.J.; Barres, B.A. Glia—More than Just Brain Glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from Brain Glue to Communication Elements: The Revolution Continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Astroglial Cradle in the Life of the Synapse. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130595. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Kanemaru, K.; Sekiya, H.; Xu, M.; Satoh, K.; Kitajima, N.; Yoshida, K.; Okubo, Y.; Sasaki, T.; Moritoh, S.; Hasuwa, H.; et al. In Vivo Visualization of Subtle, Transient, and Local Activity of Astrocytes Using an Ultrasensitive Ca2+ Indicator. Cell Rep. 2014, 8, 311–318. [Google Scholar] [CrossRef]
- Mogensen, F.L.-H.; Delle, C.; Nedergaard, M. The Glymphatic System (En)during Inflammation. Int. J. Mol. Sci. 2021, 22, 7491. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.O.; Djuanda, E.; Yow, H.-Y.; Carare, R.O. Lymphatic Drainage of the Brain and the Pathophysiology of Neurological Disease. Acta Neuropathol. 2009, 117, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Asan, E.; Püschel, B.; Kugler, P. Cellular and Regional Distribution of the Glutamate Transporter GLAST in the CNS of Rats: Nonradioactive In Situ Hybridization and Comparative Immunocytochemistry. J. Neurosci. 1997, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.M.; Sullivan, R.K.P.; Scott, H.L.; Finkelstein, D.I.; Colditz, P.B.; Lingwood, B.E.; Dodd, P.R.; Pow, D.V. Glial Glutamate Transporter Expression Patterns in Brains from Multiple Mammalian Species. Glia 2005, 49, 520–541. [Google Scholar] [CrossRef] [PubMed]
- Anlauf, E.; Derouiche, A. Glutamine Synthetase as an Astrocytic Marker: Its Cell Type and Vesicle Localization. Front. Endocrinol. 2013, 4. [Google Scholar] [CrossRef]
- Ogata, K.; Kosaka, T. Structural and Quantitative Analysis of Astrocytes in the Mouse Hippocampus. Neuroscience 2002, 113, 221–233. [Google Scholar] [CrossRef]
- Hachem, S.; Aguirre, A.; Vives, V.; Marks, A.; Gallo, V.; Legraverend, C. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia 2005, 51, 81–97. [Google Scholar] [CrossRef]
- Steiner, J.; Bernstein, H.-G.; Bielau, H.; Berndt, A.; Brisch, R.; Mawrin, C.; Keilhoff, G.; Bogerts, B. Evidence for a Wide Extra-Astrocytic Distribution of S100B in Human Brain. BMC Neurosci. 2007, 8, 2. [Google Scholar] [CrossRef]
- Cammer, W. Glutamine Synthetase in the Central Nervous System Is Not Confined to Astrocytes. J. Neuroimmunol. 1990, 26, 173–178. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, F.; Eng, L.F.; Gibbs, M.A. Glutamine Synthetase Immunoreactivity Is Present in Oligodendroglia of Various Regions of the Central Nervous System. Glia 1990, 3, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Asan, E.; Lesch, K.-P.; Kugler, P. A Splice Variant of Glutamate Transporter GLT1/EAAT2 Expressed in Neurons: Cloning and Localization in Rat Nervous System. Neuroscience 2002, 109, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Patel, D.; Ochalski, P.A.Y.; Stelmack, G.L. Connexin30 in Rodent, Cat and Human Brain: Selective Expression in Gray Matter Astrocytes, Co-Localization with Connexin43 at Gap Junctions and Late Developmental Appearance. Neuroscience 1999, 88, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Hol, E.M.; Pekny, M. Glial Fibrillary Acidic Protein (GFAP) and the Astrocyte Intermediate Filament System in Diseases of the Central Nervous System. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial Fibrillary Acidic Protein: GFAP-Thirty-One Years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Du, J.; Yi, M.; Zhou, F.; He, W.; Yang, A.; Qiu, M.; Huang, H. S100B Is Selectively Expressed by Gray Matter Protoplasmic Astrocytes and Myelinating Oligodendrocytes in the Developing CNS. Mol. Brain 2021, 14, 154. [Google Scholar] [CrossRef]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular Basis of Astrocyte Diversity and Morphology across the CNS in Health and Disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Wilson, C.L.; Hayward, S.L.; Kidambi, S. Astrogliosis in a Dish: Substrate Stiffness Induces Astrogliosis in Primary Rat Astrocytes. RSC Adv. 2016, 6, 34447–34457. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.; Katagiri, Y.; Geller, H.M. An In Vitro Model of Reactive Astrogliosis and Its Effect on Neuronal Growth. In Astrocytes: Methods and Protocols; Milner, R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 327–340. ISBN 978-1-61779-452-0. [Google Scholar]
- Badia-Soteras, A.; de Vries, J.; Dykstra, W.; Broersen, L.M.; Verkuyl, J.M.; Smit, A.B.; Verheijen, M.H.G. High-Throughput Analysis of Astrocyte Cultures Shows Prevention of Reactive Astrogliosis by the Multi-Nutrient Combination Fortasyn Connect. Cells 2022, 11, 1428. [Google Scholar] [CrossRef]
- Cullen, D.K.; Simon, C.M.; LaPlaca, M.C. Strain Rate-Dependent Induction of Reactive Astrogliosis and Cell Death in Three-Dimensional Neuronal-Astrocytic Co-Cultures. Brain Res. 2007, 1158, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Chin, L.; Georges, P.C.; Byfield, F.J.; Bucki, R.; Kim, R.; Weaver, M.; Wells, R.G.; Marcinkiewicz, C.; Janmey, P.A. Compression Stiffening of Brain and Its Effect on Mechanosensing by Glioma Cells. New J. Phys. 2014, 16, 075002. [Google Scholar] [CrossRef]
- Pekny, M.; Nilsson, M. Astrocyte Activation and Reactive Gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Rocha, A.S.; Souza, D.G.; Leffa, D.T.; De Bastiani, M.A.; Schu, G.; Lukasewicz Ferreira, P.C.; Venturin, G.T.; Greggio, S.; Ribeiro, C.T.; et al. Activated Peripheral Blood Mononuclear Cell Mediators Trigger Astrocyte Reactivity. Brain. Behav. Immun. 2019, 80, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, J.; Chen, Y.; Collier, J.M.; Capuk, O.; Jin, S.; Sun, M.; Mondal, S.K.; Whiteside, T.L.; Stolz, D.B.; et al. NOX Activation in Reactive Astrocytes Regulates Astrocytic LCN2 Expression and Neurodegeneration. Cell Death Dis. 2022, 13, 371. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Huo, J. A1/A2 Astrocytes in Central Nervous System Injuries and Diseases: Angels or Devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef]
- Bretheau, F.; Castellanos-Molina, A.; Bélanger, D.; Kusik, M.; Mailhot, B.; Boisvert, A.; Vallières, N.; Lessard, M.; Gunzer, M.; Liu, X.; et al. The Alarmin Interleukin-1α Triggers Secondary Degeneration through Reactive Astrocytes and Endothelium after Spinal Cord Injury. Nat. Commun. 2022, 13, 5786. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of Neuropathology-Associated Reactive Astrocytes: A Systematic Review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Sekar, S.; McDonald, J.; Cuyugan, L.; Aldrich, J.; Kurdoglu, A.; Adkins, J.; Serrano, G.; Beach, T.G.; Craig, D.W.; Valla, J.; et al. Alzheimer’s Disease Is Associated with Altered Expression of Genes Involved in Immune Response and Mitochondrial Processes in Astrocytes. Neurobiol. Aging 2015, 36, 583–591. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Garwood, C.J.; Pooler, A.M.; Atherton, J.; Hanger, D.P.; Noble, W. Astrocytes Are Important Mediators of Aβ-Induced Neurotoxicity and Tau Phosphorylation in Primary Culture. Cell Death Dis. 2011, 2, e167. [Google Scholar] [CrossRef]
- Bi, F.; Huang, C.; Tong, J.; Qiu, G.; Huang, B.; Wu, Q.; Li, F.; Xu, Z.; Bowser, R.; Xia, X.-G.; et al. Reactive Astrocytes Secrete Lcn2 to Promote Neuron Death. Proc. Natl. Acad. Sci. USA 2013, 110, 4069–4074. [Google Scholar] [CrossRef]
- Caldwell, A.L.M.; Sancho, L.; Deng, J.; Bosworth, A.; Miglietta, A.; Diedrich, J.K.; Shokhirev, M.N.; Allen, N.J. Aberrant Astrocyte Protein Secretion Contributes to Altered Neuronal Development in Multiple Models of Neurodevelopmental Disorders. Nat. Neurosci. 2022, 25, 1163–1178. [Google Scholar] [CrossRef]
- Ding, Z.-B.; Song, L.-J.; Wang, Q.; Kumar, G.; Yan, Y.-Q.; Ma, C.-G. Astrocytes: A Double-Edged Sword in Neurodegenerative Diseases. Neural Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [CrossRef]
- Brandebura, A.N.; Paumier, A.; Onur, T.S.; Allen, N.J. Astrocyte Contribution to Dysfunction, Risk and Progression in Neurodegenerative Disorders. Nat. Rev. Neurosci. 2023, 24, 23–39. [Google Scholar] [CrossRef]
- Edison, P. Astroglial Activation: Current Concepts and Future Directions. Alzheimers Dement. J. Alzheimers Assoc. 2024. [Google Scholar] [CrossRef] [PubMed]
- Glezer, I.; Zekki, H.; Scavone, C.; Rivest, S. Modulation of the Innate Immune Response by NMDA Receptors Has Neuropathological Consequences. J. Neurosci. 2003, 23, 11094–11103. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S.; Lachance, C.; Patrizi, S.; Lefebvre, S.; Follett, P.L.; Jensen, F.E.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. The Toll-Like Receptor TLR4 Is Necessary for Lipopolysaccharide-Induced Oligodendrocyte Injury in the CNS. J. Neurosci. 2002, 22, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Saura, J. Microglial Cells in Astroglial Cultures: A Cautionary Note. J. Neuroinflamm. 2007, 4, 26. [Google Scholar] [CrossRef]
- Gorina, R.; Font-Nieves, M.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Astrocyte TLR4 Activation Induces a Proinflammatory Environment through the Interplay between MyD88-Dependent NFκB Signaling, MAPK, and Jak1/Stat1 Pathways. Glia 2011, 59, 242–255. [Google Scholar] [CrossRef]
- Kumamaru, H.; Saiwai, H.; Kobayakawa, K.; Kubota, K.; van Rooijen, N.; Inoue, K.; Iwamoto, Y.; Okada, S. Liposomal Clodronate Selectively Eliminates Microglia from Primary Astrocyte Cultures. J. Neuroinflamm. 2012, 9, 116. [Google Scholar] [CrossRef]
- Hupp, S.; Iliev, A.I. CSF-1 Receptor Inhibition as a Highly Effective Tool for Depletion of Microglia in Mixed Glial Cultures. J. Neurosci. Methods 2020, 332, 108537. [Google Scholar] [CrossRef]
- Van Zeller, M.; Sebastião, A.M.; Valente, C.A. Microglia Depletion from Primary Glial Cultures Enables to Accurately Address the Immune Response of Astrocytes. Biomolecules 2022, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Bennett, F.C.; Tucker, A.F.; Collins, H.Y.; Mulinyawe, S.B.; Barres, B.A. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 2017, 94, 759–773.e8. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, N.J.; Benveniste, E.N. Interleukin-6 Expression and Regulation in Astrocytes. J. Neuroimmunol. 1999, 100, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Glezer, I.; Chernomoretz, A.; David, S.; Plante, M.-M.; Rivest, S. Genes Involved in the Balance between Neuronal Survival and Death during Inflammation. PLoS ONE 2007, 2, e310. [Google Scholar] [CrossRef]
- Glezer, I.; Rivest, S. Oncostatin M Is a Novel Glucocorticoid-Dependent Neuroinflammatory Factor That Enhances Oligodendrocyte Precursor Cell Activity in Demyelinated Sites. Brain Behav. Immun. 2010, 24, 695–704. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Datta Chaudhuri, A.; Dasgheyb, R.M.; DeVine, L.R.; Bi, H.; Cole, R.N.; Haughey, N.J. Stimulus-Dependent Modifications in Astrocyte-Derived Extracellular Vesicle Cargo Regulate Neuronal Excitability. Glia 2020, 68, 128–144. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.-W.; Trout, A.; Talbot, C.C.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFα and IL-1β Modify the miRNA Cargo of Astrocyte Shed Extracellular Vesicles to Regulate Neurotrophic Signaling in Neurons. Cell Death Dis. 2018, 9, 363. [Google Scholar] [CrossRef]
- Yu, T.; Wang, X.; Zhi, T.; Zhang, J.; Wang, Y.; Nie, E.; Zhou, F.; You, Y.; Liu, N. Delivery of MGMT mRNA to Glioma Cells by Reactive Astrocyte-Derived Exosomes Confers a Temozolomide Resistance Phenotype. Cancer Lett. 2018, 433, 210–220. [Google Scholar] [CrossRef]
- Freeman, L.; Guo, H.; David, C.N.; Brickey, W.J.; Jha, S.; Ting, J.P.-Y. NLR Members NLRC4 and NLRP3 Mediate Sterile Inflammasome Activation in Microglia and Astrocytes. J. Exp. Med. 2017, 214, 1351–1370. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, J.; de Rivero Vaccari, J.P.; Keane, R.W. Human Astrocytes Express a Novel NLRP2 Inflammasome. Glia 2013, 61, 1113–1121. [Google Scholar] [CrossRef]
- Liu, H.-D.; Li, W.; Chen, Z.-R.; Hu, Y.-C.; Zhang, D.-D.; Shen, W.; Zhou, M.-L.; Zhu, L.; Hang, C.-H. Expression of the NLRP3 Inflammasome in Cerebral Cortex After Traumatic Brain Injury in a Rat Model. Neurochem. Res. 2013, 38, 2072–2083. [Google Scholar] [CrossRef] [PubMed]
- Johann, S.; Heitzer, M.; Kanagaratnam, M.; Goswami, A.; Rizo, T.; Weis, J.; Troost, D.; Beyer, C. NLRP3 Inflammasome Is Expressed by Astrocytes in the SOD1 Mouse Model of ALS and in Human Sporadic ALS Patients. Glia 2015, 63, 2260–2273. [Google Scholar] [CrossRef]
- Couturier, J.; Stancu, I.-C.; Schakman, O.; Pierrot, N.; Huaux, F.; Kienlen-Campard, P.; Dewachter, I.; Octave, J.-N. Activation of Phagocytic Activity in Astrocytes by Reduced Expression of the Inflammasome Component ASC and Its Implication in a Mouse Model of Alzheimer Disease. J. Neuroinflamm. 2016, 13, 20. [Google Scholar] [CrossRef]
- Choi, J.-K.; Park, S.-Y.; Kim, K.H.; Park, S.R.; Lee, S.-G.; Choi, B.H. GM-CSF Reduces Expression of Chondroitin Sulfate Proteoglycan (CSPG) Core Proteins in TGF-β-Treated Primary Astrocytes. BMB Rep. 2014, 47, 679–684. [Google Scholar] [CrossRef]
- Schachtrup, C.; Ryu, J.K.; Helmrick, M.J.; Vagena, E.; Galanakis, D.K.; Degen, J.L.; Margolis, R.U.; Akassoglou, K. Fibrinogen Triggers Astrocyte Scar Formation by Promoting the Availability of Active TGF-β after Vascular Damage. J. Neurosci. 2010, 30, 5843–5854. [Google Scholar] [CrossRef] [PubMed]
- Gris, P.; Tighe, A.; Levin, D.; Sharma, R.; Brown, A. Transcriptional Regulation of Scar Gene Expression in Primary Astrocytes. Glia 2007, 55, 1145–1155. [Google Scholar] [CrossRef]
- Klemens, J.; Ciurkiewicz, M.; Chludzinski, E.; Iseringhausen, M.; Klotz, D.; Pfankuche, V.M.; Ulrich, R.; Herder, V.; Puff, C.; Baumgärtner, W.; et al. Neurotoxic Potential of Reactive Astrocytes in Canine Distemper Demyelinating Leukoencephalitis. Sci. Rep. 2019, 9, 11689. [Google Scholar] [CrossRef]
- Ren, Z.; Iliff, J.J.; Yang, L.; Yang, J.; Chen, X.; Chen, M.J.; Giese, R.N.; Wang, B.; Shi, X.; Nedergaard, M. ‘Hit & Run’ Model of Closed-Skull Traumatic Brain Injury (TBI) Reveals Complex Patterns of Post-Traumatic AQP4 Dysregulation. J. Cereb. Blood Flow Metab. 2013, 33, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, M.; Lehtonen, S.; Jaronen, M.; Goldsteins, G.; Hämäläinen, R.H.; Koistinaho, J. Astrocyte Alterations in Neurodegenerative Pathologies and Their Modeling in Human Induced Pluripotent Stem Cell Platforms. Cell. Mol. Life Sci. 2019, 76, 2739–2760. [Google Scholar] [CrossRef] [PubMed]
- Prah, J.; Winters, A.; Chaudhari, K.; Hersh, J.; Liu, R.; Yang, S.-H. A Novel Serum Free Primary Astrocyte Culture Method That Mimic Quiescent Astrocyte Phenotype. J. Neurosci. Methods 2019, 320, 50–63. [Google Scholar] [CrossRef]
- Taylor, X.; Cisternas, P.; You, Y.; You, Y.; Xiang, S.; Marambio, Y.; Zhang, J.; Vidal, R.; Lasagna-Reeves, C.A. A1 Reactive Astrocytes and a Loss of TREM2 Are Associated with an Early Stage of Pathology in a Mouse Model of Cerebral Amyloid Angiopathy. J. Neuroinflamm. 2020, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Nath, C.; Shukla, R. Melatonin Attenuated Mediators of Neuroinflammation and Alpha-7 Nicotinic Acetylcholine Receptor mRNA Expression in Lipopolysaccharide (LPS) Stimulated Rat Astrocytoma Cells, C6. Free Radic. Res. 2012, 46, 1167–1177. [Google Scholar] [CrossRef]
- Niranjan, R.; Nath, C.; Shukla, R. The Mechanism of Action of MPTP-Induced Neuroinflammation and Its Modulation by Melatonin in Rat Astrocytoma Cells, C6. Free Radic. Res. 2010, 44, 1304–1316. [Google Scholar] [CrossRef]
- Furihata, T.; Ito, R.; Kamiichi, A.; Saito, K.; Chiba, K. Establishment and Characterization of a New Conditionally Immortalized Human Astrocyte Cell Line. J. Neurochem. 2016, 136, 92–105. [Google Scholar] [CrossRef]
- Efremova, L.; Chovancova, P.; Adam, M.; Gutbier, S.; Schildknecht, S.; Leist, M. Switching from Astrocytic Neuroprotection to Neurodegeneration by Cytokine Stimulation. Arch. Toxicol. 2017, 91, 231–246. [Google Scholar] [CrossRef]
- Rocchio, F.; Tapella, L.; Manfredi, M.; Chisari, M.; Ronco, F.; Ruffinatti, F.A.; Conte, E.; Canonico, P.L.; Sortino, M.A.; Grilli, M.; et al. Correction: Gene Expression, Proteome and Calcium Signaling Alterations in Immortalized Hippocampal Astrocytes from an Alzheimer’s Disease Mouse Model. Cell Death Dis. 2020, 11, 1–2. [Google Scholar] [CrossRef]
- Rickner, H.D.; Jiang, L.; Hong, R.; O’Neill, N.K.; Mojica, C.A.; Snyder, B.J.; Zhang, L.; Shaw, D.; Medalla, M.; Wolozin, B.; et al. Single Cell Transcriptomic Profiling of a Neuron-Astrocyte Assembloid Tauopathy Model. Nat. Commun. 2022, 13, 6275. [Google Scholar] [CrossRef] [PubMed]
- BaofengFeng, U.; Amponsah, A.E.; Guo, R.; Liu, X.; Zhang, J.; Du, X.; Zhou, Z.; He, J.; Ma, J.; Cui, H. Autophagy-Mediated Inflammatory Cytokine Secretion in Sporadic ALS Patient iPSC-Derived Astrocytes. Oxid. Med. Cell. Longev. 2022, 2022, e6483582. [Google Scholar] [CrossRef] [PubMed]
- Kawatani, K.; Nambara, T.; Nawa, N.; Yoshimatsu, H.; Kusakabe, H.; Hirata, K.; Tanave, A.; Sumiyama, K.; Banno, K.; Taniguchi, H.; et al. A Human Isogenic iPSC-Derived Cell Line Panel Identifies Major Regulators of Aberrant Astrocyte Proliferation in Down Syndrome. Commun. Biol. 2021, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Coleman, H.A.; Meagher, L.; Forsythe, J.S.; Parkington, H.C. 3D Functional Neuronal Networks in Free-Standing Bioprinted Hydrogel Constructs. Adv. Healthc. Mater. 2023, 12, 2300801. [Google Scholar] [CrossRef] [PubMed]
- Placone, A.L.; McGuiggan, P.M.; Bergles, D.E.; Guerrero-Cazares, H.; Quiñones-Hinojosa, A.; Searson, P.C. Human Astrocytes Develop Physiological Morphology and Remain Quiescent in a Novel 3D Matrix. Biomaterials 2015, 42, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Gensini, L.; Serna, J.A.; Rubio, D.; Orozco, J.C.; Bolaños, N.I.; Cruz, J.C.; Muñoz-Camargo, C. Three-Dimensional Neuroimmune Co-Culture System for Modeling Parkinson’s Disease Microenvironmentsin Vitro. Biofabrication 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Paşca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.-Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional Cortical Neurons and Astrocytes from Human Pluripotent Stem Cells in 3D Culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Rouleau, N.; Parker, R.N.; Walsh, K.G.; Gehrke, L.; Kaplan, D.L. A 3D Human Brain-like Tissue Model of Herpes-Induced Alzheimer’s Disease. Sci. Adv. 2020, 6, eaay8828. [Google Scholar] [CrossRef]
- Schafer, S.T.; Mansour, A.A.; Schlachetzki, J.C.M.; Pena, M.; Ghassemzadeh, S.; Mitchell, L.; Mar, A.; Quang, D.; Stumpf, S.; Ortiz, I.S.; et al. An in Vivo Neuroimmune Organoid Model to Study Human Microglia Phenotypes. Cell 2023, 186, 2111–2126.e20. [Google Scholar] [CrossRef]
- Calvillo, M.; Diaz, A.; Limon, D.I.; Mayoral, M.A.; Chánez-Cárdenas, M.E.; Zenteno, E.; Montaño, L.F.; Guevara, J.; Espinosa, B. Amyloid-Β25–35 Induces a Permanent Phosphorylation of HSF-1, but a Transitory and Inflammation-Independent Overexpression of Hsp-70 in C6 Astrocytoma Cells. Neuropeptides 2013, 47, 339–346. [Google Scholar] [CrossRef]
- Dozio, V.; Sanchez, J.-C. Profiling the Proteomic Inflammatory State of Human Astrocytes Using DIA Mass Spectrometry. J. Neuroinflamm. 2018, 15, 331. [Google Scholar] [CrossRef]
- Millet, L.J.; Gillette, M.U. Over a Century of Neuron Culture: From the Hanging Drop to Microfluidic Devices. Yale J. Biol. Med. 2012, 85, 501–521. [Google Scholar] [PubMed]
- Hogue, M.J. Human Fetal Brain Cells in Tissue Cultures; Their Identification and Motility. J. Exp. Zool. 1947, 106, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Kriegstein, A.; Alvarez-Buylla, A. The Glial Nature of Embryonic and Adult Neural Stem Cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef] [PubMed]
- Shein, H.M. Propagation of Human Fetal Spongioblasts and Astrocytes in Dispersed Cell Cultures. Exp. Cell Res. 1965, 40, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Shein, H.M. Neoplastic Transformation of Hamster Astrocytes in Vitro by Simian Virus 40 and Polyoma Virus. Science 1968, 159, 1476–1477. [Google Scholar] [CrossRef] [PubMed]
- Raff, M.C.; Miller, R.H.; Noble, M. A Glial Progenitor Cell That Develops in Vitro into an Astrocyte or an Oligodendrocyte Depending on Culture Medium. Nature 1983, 303, 390–396. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.D.; de Vellis, J. Preparation of Separate Astroglial and Oligodendroglial Cell Cultures from Rat Cerebral Tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef]
- Booher, J.; Sensenbrenner, M. Growth and Cultivation of Dissociated Neurons and Glial Cells from Embryonic Chick, Rat and Human Brain in Flask Cultures. Neurobiology 1972, 2, 97–105. [Google Scholar]
- Vellis, J.D.; Cole, R. Preparation of Mixed Glial Cultures from Postnatal Rat Brain. Methods Mol. Biol. Clifton NJ 2012, 814, 49–59. [Google Scholar] [CrossRef]
- Bouvier, M.; Szatkowski, M.; Amato, A.; Attwell, D. The Glial Cell Glutamate Uptake Carrier Countertransports pH-Changing Anions. Nature 1992, 360, 471–474. [Google Scholar] [CrossRef]
- Kimelberg, H.K. Primary Astrocyte Cultures—A Key to Astrocyte Function. Cell. Mol. Neurobiol. 1983, 3, 1–16. [Google Scholar] [CrossRef]
- Schousboe, A.; Svenneby, G.; Hertz, L. Uptake and Metabolism of Glutamate in Astrocytes Cultured from Dissociated Mouse Brain Hemispheres. J. Neurochem. 1977, 29, 999–1005. [Google Scholar] [CrossRef]
- Friede, R.L. The Enzymatic Response of Astrocytes to Various Ions In Vitro. J. Cell Biol. 1964, 20, 5–15. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate Uptake into Astrocytes Stimulates Aerobic Glycolysis: A Mechanism Coupling Neuronal Activity to Glucose Utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Struckhoff, G. Cocultures of Meningeal and Astrocytic Cells—A Model for the Formation of the Glial-Limiting Membrane. Int. J. Dev. Neurosci. 1995, 13, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Yamasaki, R.; Takase, E.O.; Iida, K.; Watanabe, M.; Masaki, K.; Wijering, M.H.C.; Yamaguchi, H.; Kira, J.-I.; Isobe, N. Iguratimod Ameliorates the Severity of Secondary Progressive Multiple Sclerosis in Model Mice by Directly Inhibiting IL-6 Production and Th17 Cell Migration via Mitigation of Glial Inflammation. Biology 2023, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Szpakowski, P.; Ksiazek-Winiarek, D.; Czpakowska, J.; Kaluza, M.; Milewska-Jedrzejczak, M.; Glabinski, A. Astrocyte-Derived Exosomes Differentially Shape T Cells’ Immune Response in MS Patients. Int. J. Mol. Sci. 2023, 24, 7470. [Google Scholar] [CrossRef] [PubMed]
- Corvace, F.; Faustmann, T.J.; Heckers, S.; Faustmann, P.M.; Ismail, F.S. Experimental Investigations of Monomethyl and Dimethyl Fumarate in an Astrocyte-Microglia Co-Culture Model of Inflammation. Pharmacology 2023, 108, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Bobermin, L.D.; Souza, D.G.; Bellaver, B.; Gonçalves, C.-A.; Souza, D.O. Guanosine Protects C6 Astroglial Cells against Azide-Induced Oxidative Damage: A Putative Role of Heme Oxygenase 1. J. Neurochem. 2014, 130, 61–74. [Google Scholar] [CrossRef]
- Arús, B.A.; Souza, D.G.; Bellaver, B.; Souza, D.O.; Gonçalves, C.-A.; Quincozes-Santos, A.; Bobermin, L.D. Resveratrol Modulates GSH System in C6 Astroglial Cells through Heme Oxygenase 1 Pathway. Mol. Cell. Biochem. 2017, 428, 67–77. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Bobermin, L.D.; Souza, D.O.; Quincozes-Santos, A. Riluzole Increases Glutamate Uptake by Cultured C6 Astroglial Cells. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2013, 31, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Wiley, C.; Randell, K.; Darling-Reed, S.F.; Latinwo, L.M.; Agharahimi, M.; Soliman, K.F.A.; Goodman, C.B. Identification of Cytotoxic Markers in Methamphetamine Treated Rat C6 Astroglia-like Cells. Sci. Rep. 2019, 9, 9412. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.A.; Harris, N.; Soliman, K.F. Food Constituents Attenuate Monoamine Oxidase Activity and Peroxide Levels in C6 Astrocyte Cells. Planta Med. 1998, 64, 603–606. [Google Scholar] [CrossRef]
- Galland, F.; Seady, M.; Taday, J.; Smaili, S.S.; Gonçalves, C.A.; Leite, M.C. Astrocyte Culture Models: Molecular and Function Characterization of Primary Culture, Immortalized Astrocytes and C6 Glioma Cells. Neurochem. Int. 2019, 131, 104538. [Google Scholar] [CrossRef]
- Sibenaller, Z.A.; Etame, A.B.; Ali, M.M.; Barua, M.; Braun, T.A.; Casavant, T.L.; Ryken, T.C. Genetic Characterization of Commonly Used Glioma Cell Lines in the Rat Animal Model System. Neurosurg. Focus 2005, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vries, G.H.D.; Boullerne, A.I. Glial Cell Lines: An Overview. Neurochem. Res. 2010, 35, 1978–2000. [Google Scholar] [CrossRef]
- Urati, A.; Dey, M.; Gautam, A.S.; Singh, R.K. Iron-Induced Cellular in Vitro Neurotoxic Responses in Rat C6 Cell Line. Environ. Toxicol. 2022, 37, 1968–1978. [Google Scholar] [CrossRef]
- Major, E.O.; Miller, A.E.; Mourrain, P.; Traub, R.G.; Widt, E.D.; Sever, J. Establishment of a Line of Human Fetal Glial Cells That Supports JC Virus Multiplication. Proc. Natl. Acad. Sci. USA 1985, 82, 1257–1261. [Google Scholar] [CrossRef]
- Prowse, K.R.; Greider, C.W. Developmental and Tissue-Specific Regulation of Mouse Telomerase and Telomere Length. Proc. Natl. Acad. Sci. USA 1995, 92, 4818–4822. [Google Scholar] [CrossRef]
- Morikawa, M.; Asai, K.; Kokubo, M.; Fujita, K.; Yoneda, K.; Yamamoto, N.; Inoue, Y.; Iida, J.; Kishimoto, T.; Kato, T. Isolation and Characterization of a New Immortal Rat Astrocyte with a High Expression of NGF mRNA. Neurosci. Res. 2001, 39, 205–212. [Google Scholar] [CrossRef]
- Frisa, P.S.; Jacobberger, J.W. Cell Density Related Gene Expression: SV40 Large T Antigen Levels in Immortalized Astrocyte Lines. BMC Cell Biol. 2002, 3, 10. [Google Scholar] [CrossRef]
- Kamiichi, A.; Furihata, T.; Kishida, S.; Ohta, Y.; Saito, K.; Kawamatsu, S.; Chiba, K. Establishment of a New Conditionally Immortalized Cell Line from Human Brain Microvascular Endothelial Cells: A Promising Tool for Human Blood–Brain Barrier Studies. Brain Res. 2012, 1488, 113–122. [Google Scholar] [CrossRef]
- Whittemore, S.R.; Neary, J.T.; Kleitman, N.; Sanon, H.R.; Benigno, A.; Donahue, R.P.; Norenberg, M.D. Isolation and Characterization of Conditionally Immortalized Astrocyte Cell Lines Derived from Adult Human Spinal Cord. Glia 1994, 10, 211–226. [Google Scholar] [CrossRef]
- Price, T.N.C.; Burke, J.F.; Mayne, L.V. A Novel Human Astrocyte Cell Line (A735) with Astrocyte-Specific Neurotransmitter Function. Vitro Cell. Dev. Biol.-Anim. 1999, 35, 279–288. [Google Scholar] [CrossRef]
- Sonoda, Y.; Ozawa, T.; Hirose, Y.; Aldape, K.D.; McMahon, M.; Berger, M.S.; Pieper, R.O. Formation of Intracranial Tumors by Genetically Modified Human Astrocytes Defines Four Pathways Critical in the Development of Human Anaplastic Astrocytoma. Cancer Res. 2001, 61, 4956–4960. [Google Scholar]
- Sasai, K.; Akagi, T.; Aoyanagi, E.; Tabu, K.; Kaneko, S.; Tanaka, S. O6-Methylguanine-DNA Methyltransferase Is Downregulated in Transformed Astrocyte Cells: Implications for Anti-Glioma Therapies. Mol. Cancer 2007, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Alliot, F.; Pessac, B. Astrocytic Cell Clones Derived from Established Cultures of 8-Day Postnatal Mouse Cerebella. Brain Res. 1984, 306, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Kim, H. Characterization of a Microfluidic in Vitro Model of the Blood-Brain Barrier (μBBB). Lab. Chip 2012, 12, 1784–1792. [Google Scholar] [CrossRef]
- Ito, R.; Umehara, K.; Suzuki, S.; Kitamura, K.; Nunoya, K.-I.; Yamaura, Y.; Imawaka, H.; Izumi, S.; Wakayama, N.; Komori, T.; et al. A Human Immortalized Cell-Based Blood-Brain Barrier Triculture Model: Development and Characterization as a Promising Tool for Drug-Brain Permeability Studies. Mol. Pharm. 2019, 16, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Isogai, R.; Morio, H.; Okamoto, A.; Kitamura, K.; Furihata, T. Generation of a Human Conditionally Immortalized Cell-Based Multicellular Spheroidal Blood-Brain Barrier Model for Permeability Evaluation of Macromolecules. Bio-Protocol 2022, 12, e4465. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Patel, G.C.; Mao, W.; Clark, A.F. Establishment of a Conditionally Immortalized Mouse Optic Nerve Astrocyte Line. Exp. Eye Res. 2018, 176, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jat, P.S.; Noble, M.D.; Ataliotis, P.; Tanaka, Y.; Yannoutsos, N.; Larsen, L.; Kioussis, D. Direct Derivation of Conditionally Immortal Cell Lines from an H-2Kb-tsA58 Transgenic Mouse. Proc. Natl. Acad. Sci. USA 1991, 88, 5096–5100. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Toida, A.; Horiuchi, Y.; Watanabe, S.; Sasahara, M.; Kawaguchi, K.; So, T.; Imanaka, T. Generation of an Immortalized Astrocytic Cell Line from Abcd1-Deficient H-2KbtsA58 Mice to Facilitate the Study of the Role of Astrocytes in X-Linked Adrenoleukodystrophy. Heliyon 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Krencik, R.; Weick, J.P.; Liu, Y.; Zhang, Z.-J.; Zhang, S.-C. Specification of Transplantable Astroglial Subtypes from Human Pluripotent Stem Cells. Nat. Biotechnol. 2011, 29, 528–534. [Google Scholar] [CrossRef]
- Caiazzo, M.; Giannelli, S.; Valente, P.; Lignani, G.; Carissimo, A.; Sessa, A.; Colasante, G.; Bartolomeo, R.; Massimino, L.; Ferroni, S.; et al. Direct Conversion of Fibroblasts into Functional Astrocytes by Defined Transcription Factors. Stem Cell Rep. 2015, 4, 25–36. [Google Scholar] [CrossRef]
- Haidet-Phillips, A.M.; Roybon, L.; Gross, S.K.; Tuteja, A.; Donnelly, C.J.; Richard, J.-P.; Ko, M.; Sherman, A.; Eggan, K.; Henderson, C.E.; et al. Gene Profiling of Human Induced Pluripotent Stem Cell-Derived Astrocyte Progenitors Following Spinal Cord Engraftment. Stem Cells Transl. Med. 2014, 3, 575–585. [Google Scholar] [CrossRef] [PubMed]
- McGivern, J.V.; Patitucci, T.N.; Nord, J.A.; Barabas, M.-E.A.; Stucky, C.L.; Ebert, A.D. Spinal Muscular Atrophy Astrocytes Exhibit Abnormal Calcium Regulation and Reduced Growth Factor Production. Glia 2013, 61, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Serio, A.; Bilican, B.; Barmada, S.J.; Ando, D.M.; Zhao, C.; Siller, R.; Burr, K.; Haghi, G.; Story, D.; Nishimura, A.L.; et al. Astrocyte Pathology and the Absence of Non-Cell Autonomy in an Induced Pluripotent Stem Cell Model of TDP-43 Proteinopathy. Proc. Natl. Acad. Sci. USA 2013, 110, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Shaltouki, A.; Peng, J.; Liu, Q.; Rao, M.S.; Zeng, X. Efficient Generation of Astrocytes from Human Pluripotent Stem Cells in Defined Conditions. Stem Cells 2013, 31, 941–952. [Google Scholar] [CrossRef]
- Chaboub, L.S.; Deneen, B. Astrocyte Form and Function in the Developing Central Nervous System. Semin. Pediatr. Neurol. 2013, 20, 230–235. [Google Scholar] [CrossRef]
- Yuan, S.H.; Martin, J.; Elia, J.; Flippin, J.; Paramban, R.I.; Hefferan, M.P.; Vidal, J.G.; Mu, Y.; Killian, R.L.; Israel, M.A.; et al. Cell-Surface Marker Signatures for the Isolation of Neural Stem Cells, Glia and Neurons Derived from Human Pluripotent Stem Cells. PLOS ONE 2011, 6, e17540. [Google Scholar] [CrossRef]
- Tcw, J.; Wang, M.; Pimenova, A.A.; Bowles, K.R.; Hartley, B.J.; Lacin, E.; Machlovi, S.I.; Abdelaal, R.; Karch, C.M.; Phatnani, H.; et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 600–614. [Google Scholar] [CrossRef]
- Xu, X.; Lei, Y.; Luo, J.; Wang, J.; Zhang, S.; Yang, X.-J.; Sun, M.; Nuwaysir, E.; Fan, G.; Zhao, J.; et al. Prevention of β-Amyloid Induced Toxicity in Human iPS Cell-Derived Neurons by Inhibition of Cyclin-Dependent Kinases and Associated Cell Cycle Events. Stem Cell Res. 2013, 10, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Höing, S.; Rudhard, Y.; Reinhardt, P.; Glatza, M.; Stehling, M.; Wu, G.; Peiker, C.; Böcker, A.; Parga, J.A.; Bunk, E.; et al. Discovery of Inhibitors of Microglial Neurotoxicity Acting Through Multiple Mechanisms Using a Stem-Cell-Based Phenotypic Assay. Cell Stem Cell 2012, 11, 620–632. [Google Scholar] [CrossRef]
- Burkhardt, M.F.; Martinez, F.J.; Wright, S.; Ramos, C.; Volfson, D.; Mason, M.; Garnes, J.; Dang, V.; Lievers, J.; Shoukat-Mumtaz, U.; et al. A Cellular Model for Sporadic ALS Using Patient-Derived Induced Pluripotent Stem Cells. Mol. Cell. Neurosci. 2013, 56, 355–364. [Google Scholar] [CrossRef]
- Leventoux, N.; Morimoto, S.; Imaizumi, K.; Sato, Y.; Takahashi, S.; Mashima, K.; Ishikawa, M.; Sonn, I.; Kondo, T.; Watanabe, H.; et al. Human Astrocytes Model Derived from Induced Pluripotent Stem Cells. Cells 2020, 9, 2680. [Google Scholar] [CrossRef]
- Namihira, M.; Kohyama, J.; Abematsu, M.; Nakashima, K. Epigenetic Mechanisms Regulating Fate Specification of Neural Stem Cells. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2099–2109. [Google Scholar] [CrossRef]
- Yasui, T.; Uezono, N.; Nakashima, H.; Noguchi, H.; Matsuda, T.; Noda-Andoh, T.; Okano, H.; Nakashima, K. Hypoxia Epigenetically Confers Astrocytic Differentiation Potential on Human Pluripotent Cell-Derived Neural Precursor Cells. Stem Cell Rep. 2017, 8, 1743–1756. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Stathakos, P.; Caldwell, M.A.; Lane, J.D. Induced Pluripotent Stem Cell Neuronal Models for the Study of Autophagy Pathways in Human Neurodegenerative Disease. Cells 2017, 6, 24. [Google Scholar] [CrossRef]
- Moon, S.B.; Kim, D.Y.; Ko, J.-H.; Kim, Y.-S. Recent Advances in the CRISPR Genome Editing Tool Set. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Mei, A.; Marchetto, M.C. Generation of Inflammation-Responsive Astrocytes from Glial Progenitors Derived from Human Pluripotent Stem Cells. STAR Protoc. 2022, 3, 101261. [Google Scholar] [CrossRef] [PubMed]
- Kerkering, J.; Muinjonov, B.; Rosiewicz, K.S.; Diecke, S.; Biese, C.; Schiweck, J.; Chien, C.; Zocholl, D.; Conrad, T.; Paul, F.; et al. iPSC-Derived Reactive Astrocytes from Patients with Multiple Sclerosis Protect Cocultured Neurons in Inflammatory Conditions. J. Clin. Investig. 2023, 133, e164637. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Bassani, C.; De Angelis, A.; Ruffini, F.; Ottoboni, L.; Comi, G.; Martino, G.; Farina, C. Siponimod (BAF312) Activates Nrf2 While Hampering NFκB in Human Astrocytes, and Protects From Astrocyte-Induced Neurodegeneration. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Colombo, E.; Pascente, R.; Triolo, D.; Bassani, C.; De Angelis, A.; Ruffini, F.; Ottoboni, L.; Comi, G.; Martino, G.; Farina, C. Laquinimod Modulates Human Astrocyte Function and Dampens Astrocyte-Induced Neurotoxicity during Inflammation. Molecules 2020, 25, 5403. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Leng, K.; Park, J.; Sorets, A.G.; Kim, S.; Shostak, A.; Embalabala, R.J.; Mlouk, K.; Katdare, K.A.; Rose, I.V.L.; et al. Reactive Astrocytes Transduce Inflammation in a Blood-Brain Barrier Model through a TNF-STAT3 Signaling Axis and Secretion of Alpha 1-Antichymotrypsin. Nat. Commun. 2022, 13, 6581. [Google Scholar] [CrossRef] [PubMed]
- Maclean, F.L.; Williams, R.J.; Horne, M.K.; Nisbet, D.R. A Commentary on the Need for 3D-Biologically Relevant In Vitro Environments to Investigate Astrocytes and Their Role in Central Nervous System Inflammation. Neurochem. Res. 2016, 41, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension – How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef]
- Lovett, M.L.; Nieland, T.J.F.; Dingle, Y.-T.L.; Kaplan, D.L. Innovations in 3D Tissue Models of Human Brain Physiology and Diseases. Adv. Funct. Mater. 2020, 30, 1909146. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, S.; Anand, S.; Shah, T.; Tasoglu, S. Bioprinting for Neural Tissue Engineering. Trends Neurosci. 2018, 41, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Czell, D. Genetics of Amyotrophic Lateral Sclerosis. Praxis 2019, 108, 37–44. [Google Scholar] [CrossRef]
- Warren, D.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. Engineering in Vitro Human Neural Tissue Analogs by 3D Bioprinting and Electrostimulation. APL Bioeng. 2021, 5, 020901. [Google Scholar] [CrossRef]
- Führmann, T.; Hillen, L.M.; Montzka, K.; Wöltje, M.; Brook, G.A. Cell-Cell Interactions of Human Neural Progenitor-Derived Astrocytes within a Microstructured 3D-Scaffold. Biomaterials 2010, 31, 7705–7715. [Google Scholar] [CrossRef]
- Fang, A.; Li, D.; Hao, Z.; Wang, L.; Pan, B.; Gao, L.; Qu, X.; He, J. Effects of Astrocyte on Neuronal Outgrowth in a Layered 3D Structure. Biomed. Eng. OnLine 2019, 18, 74. [Google Scholar] [CrossRef]
- Frampton, J.P.; Hynd, M.R.; Shuler, M.L.; Shain, W. Fabrication and Optimization of Alginate Hydrogel Constructs for Use in 3D Neural Cell Culture. Biomed. Mater. 2011, 6, 15002. [Google Scholar] [CrossRef]
- Matthiesen, I.; Jury, M.; Boroojeni, F.R.; Ludwig, S.L.; Holzreuter, M.; Buchmann, S.; Träger, A.Å.; Selegård, R.; Winkler, T.E.; Aili, D.; et al. Astrocyte 3D Culture and Bioprinting Using Peptide Functionalized Hyaluronan Hydrogels. Sci. Technol. Adv. Mater. 2023, 24, 2165871. [Google Scholar] [CrossRef]
- O’Donnell, J.C.; Katiyar, K.S.; Panzer, K.V.; Cullen, D.K. A Tissue-Engineered Rostral Migratory Stream for Directed Neuronal Replacement. Neural Regen. Res. 2018, 13, 1327–1331. [Google Scholar] [CrossRef]
- Seidlits, S.K.; Khaing, Z.Z.; Petersen, R.R.; Nickels, J.D.; Vanscoy, J.E.; Shear, J.B.; Schmidt, C.E. The Effects of Hyaluronic Acid Hydrogels with Tunable Mechanical Properties on Neural Progenitor Cell Differentiation. Biomaterials 2010, 31, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Fitzgerald, W.; Liu, Q.-Y.; O’Shaughnessy, T.J.; Maric, D.; Lin, H.J.; Alkon, D.L.; Barker, J.L. CNS Stem and Progenitor Cell Differentiation into Functional Neuronal Circuits in Three-Dimensional Collagen Gels. Exp. Neurol. 2004, 190, 276–288. [Google Scholar] [CrossRef]
- Batenburg, K.L.; Sestito, C.; Cornelissen-Steijger, P.; Weering, J.R.T.V.; Price, L.S.; Heine, V.M.; Scheper, W. A 3D Human Co-Culture to Model Neuron-Astrocyte Interactions in Tauopathies. Biol. Proced. Online 2023, 25, 4. [Google Scholar] [CrossRef]
- Prasannan, P.; Siney, E.; Chatterjee, S.; Johnston, D.; Shah, M.; Mudher, A.; Willaime-Morawek, S. A 3D-Induced Pluripotent Stem Cell-Derived Human Neural Culture Model to Study Certain Molecular and Biochemical Aspects of Alzheimer’s Disease. Vitro Models 2022, 1, 447–462. [Google Scholar] [CrossRef]
- East, E.; Golding, J.P.; Phillips, J.B. A Versatile 3D Culture Model Facilitates Monitoring of Astrocytes Undergoing Reactive Gliosis. J. Tissue Eng. Regen. Med. 2009, 3, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.M.D.; Kavanagh, E.; Allenby, G.; Vassey, M. Bioengineered 3D Glial Cell Culture Systems and Applications for Neurodegeneration and Neuroinflammation. SLAS Discov. 2017, 22, 583–601. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Mundim, M.V.; Lemes, R.M.R.; Cruz, E.M.; Ribeiro, T.N.; Santiago, C.F.; da Fonsêca, J.H.L.; Benincasa, J.C.; Stilhano, R.S.; Mantovani, N.; et al. 3D Bioprinted Neural-Like Tissue as a Platform to Study Neurotropism of Mouse-Adapted SARS-CoV-2. Adv. Biol. 2022, 6, 2200002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Long, X.; Xu, T. Three-Dimensional-Engineered Bioprinted in Vitro Human Neural Stem Cell Self-Assembling Culture Model Constructs of Alzheimer’s Disease. Bioact. Mater. 2022, 11, 192–205. [Google Scholar] [CrossRef]
- Brewer, G.J.; Torricelli, J.R. Isolation and Culture of Adult Neurons and Neurospheres. Nat. Protoc. 2007, 2, 1490–1498. [Google Scholar] [CrossRef]
- Gage, F.H.; Coates, P.W.; Palmer, T.D.; Kuhn, H.G.; Fisher, L.J.; Suhonen, J.O.; Peterson, D.A.; Suhr, S.T.; Ray, J. Survival and Differentiation of Adult Neuronal Progenitor Cells Transplanted to the Adult Brain. Proc. Natl. Acad. Sci. USA 1995, 92, 11879–11883. [Google Scholar] [CrossRef]
- Jensen, J.B.; Parmar, M. Strengths and Limitations of the Neurosphere Culture System. Mol. Neurobiol. 2006, 34, 153–161. [Google Scholar] [CrossRef]
- Shou, Y.; Liang, F.; Xu, S.; Li, X. The Application of Brain Organoids: From Neuronal Development to Neurological Diseases. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Sun, N.; Meng, X.; Liu, Y.; Song, D.; Jiang, C.; Cai, J. Applications of Brain Organoids in Neurodevelopment and Neurological Diseases. J. Biomed. Sci. 2021, 28, 30. [Google Scholar] [CrossRef]
- Mayhew, C.N.; Singhania, R. A Review of Protocols for Brain Organoids and Applications for Disease Modeling. STAR Protoc. 2023, 4, 101860. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-Bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent Progress in Translational Engineered in Vitro Models of the Central Nervous System. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-W.; Hsu, S. Using 3D Bioprinting to Produce Mini-Brain. Neural Regen. Res. 2017, 12, 1595. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.-J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.-S.; et al. Engineering of Human Brain Organoids with a Functional Vascular-like System. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.-N.; Jin, Y.; An, Y.; Kim, J.; Choi, Y.S.; Lee, J.S.; Kim, J.; Choi, W.-Y.; Koo, D.-J.; Yu, W.; et al. Microfluidic Device with Brain Extracellular Matrix Promotes Structural and Functional Maturation of Human Brain Organoids. Nat. Commun. 2021, 12, 4730. [Google Scholar] [CrossRef]
- Ormel, P.R.; Vieira de Sá, R.; van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis van Berlekom, A.; et al. Microglia Innately Develop within Cerebral Organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar] [CrossRef]
- Hong, Y.; Dong, X.; Chang, L.; Xie, C.; Chang, M.; Aguilar, J.S.; Lin, J.; Lin, J.; Li, Q.Q. Microglia-Containing Cerebral Organoids Derived from Induced Pluripotent Stem Cells for the Study of Neurological Diseases. iScience 2023, 26, 106267. [Google Scholar] [CrossRef]
- Sabate-Soler, S.; Nickels, S.L.; Saraiva, C.; Berger, E.; Dubonyte, U.; Barmpa, K.; Lan, Y.J.; Kouno, T.; Jarazo, J.; Robertson, G.; et al. Microglia Integration into Human Midbrain Organoids Leads to Increased Neuronal Maturation and Functionality. Glia 2022, 70, 1267–1288. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Tiwari, S.K.; Agrawal, K.; Hui, H.; Qin, Y.; Rana, T.M. Glial Cell Diversity and Methamphetamine-Induced Neuroinflammation in Human Cerebral Organoids. Mol. Psychiatry 2021, 26, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.R.; Bignami, A.; Dahl, D. Glial Fibrillary Acidic Protein in Monolayer Cultures of C-6 Glioma Cells: Effect of Aging and Dibutyryl Cyclic AMP. Brain Res. 1980, 200, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, K.; Moriya, Y.; Furuta, T.; Ohnishi, R.; Nishimoto, A. S-100 Protein in Human Glial Tumours. Acta Neurochir. 1982, 65, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.K.; Entwistle, A.; Jat, P.S.; Noble, M. The Characterization of Astrocyte Cell Lines That Display Properties of Glial Scar Tissue. Dev. Biol. 1993, 159, 87–104. [Google Scholar] [CrossRef]


| Culture Type Cell Line | Main Advantage | Limitations |
|---|---|---|
| Primary culture | Low cost and simple methodology. Disseminated methodology that is comparable to literature. | Usually employs serum (not obligatory) and is susceptible to induction of reactive phenotypes. High percentage of cellular senescence with passages; low proliferation; difficult to scale up; prone to contamination. |
| Cell lines | Easy to obtain (many are commercial); expandable; allow several passages; easy to manipulate; reduces animal usage | Cancer cell phenotype; expression profile differs from in vivo astrocytes. |
| Immortalized astrocytes | Intended to keep primary cell characteristics; less cell death and increased resilience to passages, freezing/thaw; easier to manipulate | Similar to primary cell culture; genomic integration that promotes cellular changes |
| iPSC-derived astrocyte | Relevant to disease studies (derived from patient); can be grafted; more similar to in vivo; reduces animal usage; good proliferation; allows differentiation into astrocyte types; serum-free | High cost; long-term culture; can present mutations |
| Bioprinting | Robotized; standardized dimensions; improves ECM | Expensive; delicate constructs; demands bioink characterization |
| 3D culture/organoids | Allows the analysis of complex interactions between different cell types; mimics in vivo conditions; cell heterogeneity | High cost; difficult to reproduce results, cell death in the organoid core; difficult nutrient diffusion |
| Astrocyte-Related Readouts | Primary Culture | Cell Lines | Immortalized Astrocytes | iPSC-Derived Astrocyte | Bioprinting | 3D Culture and Organoids |
|---|---|---|---|---|---|---|
| GFAP, S100B, Vimentin | [88,89,90,91,92,111,112] | [113,114] | [115,116,117] | [118,119,120] | [121,122,123] | [124] (GFAP only), [125] |
| C3, AQP4, ALDH1L1, CD44 | [92,111,112] | - | [115,117] | [118,119,120] | - | [126] |
| GS, GLT-1, GLAST | [111] | - | [115,117] | [118] | - | [126] |
| Metabolic assays (e.g., glutamate clearance/uptake, lactate and glucose uptake) | [111] | - | [115] | - | - | - |
| Pro-inflammatory (e.g., NFkB, TNF-α, IL-1 α, IL-1 β, IL-6) | [87,88,91,93] | [113,114,127] | [116,128] | [119] | [123] | [126] |
| Anti-inflammatory (e.g., IL-10) | - | - | - | - | - | [126] |
| Oxidative stress (e.g., ROS production, iNOS, DDIT3/CHOP *) | - | [113,114,127] | [116] | - | - | - |
| Intracellular calcium | - | [113,114] | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preato, A.M.; Pinheiro, E.d.S.; Rosenstock, T.R.; Glezer, I. The Relevance of Astrocytic Cell Culture Models for Neuroinflammation in Neurodegeneration Research. Neuroglia 2024, 5, 27-49. https://doi.org/10.3390/neuroglia5010003
Preato AM, Pinheiro EdS, Rosenstock TR, Glezer I. The Relevance of Astrocytic Cell Culture Models for Neuroinflammation in Neurodegeneration Research. Neuroglia. 2024; 5(1):27-49. https://doi.org/10.3390/neuroglia5010003
Chicago/Turabian StylePreato, André Maciel, Ester da Silva Pinheiro, Tatiana Rosado Rosenstock, and Isaias Glezer. 2024. "The Relevance of Astrocytic Cell Culture Models for Neuroinflammation in Neurodegeneration Research" Neuroglia 5, no. 1: 27-49. https://doi.org/10.3390/neuroglia5010003
APA StylePreato, A. M., Pinheiro, E. d. S., Rosenstock, T. R., & Glezer, I. (2024). The Relevance of Astrocytic Cell Culture Models for Neuroinflammation in Neurodegeneration Research. Neuroglia, 5(1), 27-49. https://doi.org/10.3390/neuroglia5010003