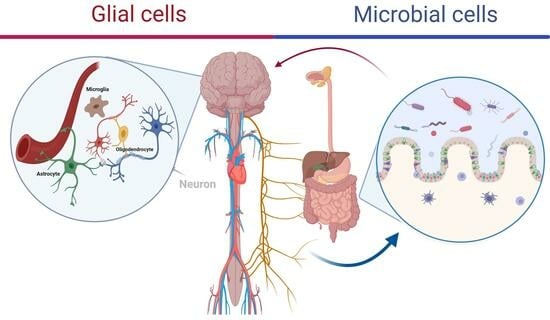
Microbiome-Glia Crosstalk: Bridging the Communication Divide in the Central Nervous System
Abstract
:1. Introduction
2. Microglia: Guardians of Neural Health
2.1. Microorganisms and Microglial States
2.2. Pattern Recognition Receptors in Microglia
2.3. Neuroinflammatory Responses and Microglia
2.4. The Role of Microglia in the Crosstalk in the Microbiome–Brain Axis
3. Astrocytes: Orchestrators of Homeostasis
3.1. Sensing and Responding to Bacterial Metabolites
3.2. Contribution to the Regulation of Neuroinflammation
3.3. Astrocytic Interactions with Microorganisms at the Blood–Brain Barrier
3.4. The Role of Astrocytes in the Crosstalk in the Microbiome-Brain Axis
4. Oligodendrocytes: Guardians of Axial Integrity in the CNS Microbial Communication
4.1. Oligodendrocyte Sensing of Bacterial Signals
4.2. Oligodendrocytes in the Crosstalk in the Microbiome–Brain Axis
4.3. Crosstalk among Oligodendrocytes, Microglia, and Microorganisms
4.4. Microbe–Oligodendrocyte Interactions in Neurodegenerative Diseases
5. Neurotransmitter Modulation in Glia–Microbe Interactions
6. Implications for Neurological Disorders
7. Implications for Health and Disease
8. Future Directions
Funding
Conflicts of Interest
References
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS Immune Privilege: Hiding in Plain Sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef]
- Parpura, V.; Basarsky, T.A.; Liu, F.; Jeftinija, K.K.; Jeftinija, S.; Haydon, P.G. Glutamate-Mediated Astrocyte-Neuron Signalling. Lett. Nat. 1994, 369, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Xu, Q.; Arcuino, G.; Kang, J.; Nedergaard, M. Astrocyte-Mediated Activation of Neuronal Kainate Receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 3172–3177. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.A.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins Are Astrocyte-Secreted Proteins That Promote CNS Synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Kucukdereli, H.; Allen, N.J.; Lee, A.T.; Feng, A.; Ozlu, M.I.; Conatser, L.M.; Chakraborty, C.; Workman, G.; Weaver, M.; Sage, E.H.; et al. Control of Excitatory CNS Synaptogenesis by Astrocyte-Secreted Proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA 2011, 108, E440–E449. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in Silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Sanmarco, L.M.; Wheeler, M.A.; Gutiérrez-Vázquez, C.; Polonio, C.M.; Linnerbauer, M.; Pinho-Ribeiro, F.A.; Li, Z.; Giovannoni, F.; Batterman, K.V.; Scalisi, G.; et al. Gut-Licensed IFNγ+ NK Cells Drive LAMP1+TRAIL+Anti-Inflammatory Astrocytes. Nature 2021, 590, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Kondakova, E.V.; Sarimov, R.M.; Yarkov, R.S.; Franceschi, C.; Vedunova, M.V. An Emerging Role of Astrocytes in Aging/Neuroinflammation and Gut-Brain Axis with Consequences on Sleep and Sleep Disorders. Ageing Res. Rev. 2023, 83, 101775. [Google Scholar] [CrossRef] [PubMed]
- Barroso, A.; Mahler, J.V.; Fonseca-Castro, P.H.; Quintana, F.J. The Aryl Hydrocarbon Receptor and the Gut–Brain Axis. Cell Mol. Immunol. 2021, 18, 259–268. [Google Scholar] [CrossRef]
- Zhou, L.; Foster, J.A. Psychobiotics and the Gut–Brain Axis: In the Pursuit of Happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Willyard, C. How Gut Microbes Could Drive Brain Disorders. Nature 2021, 590, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.; Cryan, J.F.; Paul Ross, R.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial Neuroactive Compounds Produced by Psychobiotics. Adv. Exp. Med. Biol. 2014, 817, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite Synapses: Glia, the Unacknowledged Partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Barres, B.A. Glia—More than Just Brain Glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Barres, B.A. Signaling between Glia and Neurons: Focus on Synaptic Plasticity. Curr. Opin. Neurobiol. 2005, 15, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Ransom, B.; Goldman, S.A. New Roles for Astrocytes: Redefining the Functional Architecture of the Brain. Trends Neurosci. 2003, 26, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef]
- Rothhammer, V.; Kenison, J.E.; Li, Z.; Tjon, E.; Takenaka, M.C.; Chao, C.C.; Alves De Lima, K.; Borucki, D.M.; Kaye, J.; Quintana, F.J. Aryl Hydrocarbon Receptor Activation in Astrocytes by Laquinimod Ameliorates Autoimmune Inflammation in the CNS. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e946. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and Differentiation of Microglia. Front. Cell Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Brioschi, S.; Zhou, Y.; Colonna, M. Brain Macrophages in Development, Homeostasis and Disease. J. Immunol. 2020, 204, 294. [Google Scholar] [CrossRef] [PubMed]
- Tizabi, Y.; Getachew, B.; Tsytsarev, V.; Csoka, A.B.; Copeland, R.L.; Heinbockel, T.; Tizabi, Y.; Getachew, B.; Tsytsarev, V.; Csoka, A.B.; et al. Central Nicotinic and Muscarinic Receptors in Health and Disease. In Acetylcholine—Recent Advances and New Perspectives; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Del Bigio, M.R. Ependymal Cells: Biology and Pathology. Acta Neuropathol. 2010, 119, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Götz, M. Radial Glia: Multi-Purpose Cells for Vertebrate Brain Development. Trends Neurosci. 2002, 25, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Weissman, T.A.; Riquelme, P.A.; Ivic, L.; Flint, A.C.; Kriegstein, A.R. Calcium Waves Propagate through Radial Glial Cells and Modulate Proliferation in the Developing Neocortex. Neuron 2004, 43, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Wei, D.-N.; Tang, Y. Gut Microbiota Regulate Astrocytic Functions in the Brain: Possible Therapeutic Consequences. Curr. Neuropharmacol. 2021, 19, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Rizor, A.; Pajarillo, E.; Johnson, J.; Aschner, M.; Lee, E. Astrocytic Oxidative/Nitrosative Stress Contributes to Parkinson’s Disease Pathogenesis: The Dual Role of Reactive Astrocytes. Antioxidants 2019, 8, 265. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Johnson, M.B.; Marriott, I. Murine Astrocytes Are Responsive to the Pro-Inflammatory Effects of IL-20. Neurosci. Lett. 2019, 708, 134334. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Keogh, C.E.; Kim, D.H.J.; Pusceddu, M.M.; Knotts, T.A.; Rabasa, G.; Sladek, J.A.; Hsieh, M.T.; Honeycutt, M.; Brust-Mascher, I.; Barboza, M.; et al. Myelin as a Regulator of Development of the Microbiota-Gut-Brain Axis. Brain Behav. Immun. 2021, 91, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Bostancıklıoğlu, M.; Kaplan, D.S.; Temiz, E.; Yiğit, E. Local Myelin Damage in the Hippocampus Fluctuates Gut Microbiome Profile and Memory. J. Psychiatr. Res. 2023, 158, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, V.L.; Nikonenko, I.R.; Skibo, G.G.; McKanna, J.A. Distribution of Microglia and Astrocytes in Different Regions of the Normal Adult Rat Brain. Neurophysiology 1997, 29, 343–351. [Google Scholar] [CrossRef]
- Yang, T.T.; Lin, C.; Hsu, C.T.; Wang, T.F.; Ke, F.Y.; Kuo, Y.M. Differential Distribution and Activation of Microglia in the Brain of Male C57BL/6J Mice. Brain Struct. Funct. 2013, 218, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.I. Gene Expression Profiles of M1 and M2 Microglia Characterized by Comparative Analysis of Public Datasets. Clin. Exp. Neuroimmunol. 2018, 9, 124–138. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Cells Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.N.; Douglas Fields, R. Regulation of Myelination by Microglia. Sci. Adv. 2021, 7, 1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, J.; Zhang, Y.; Huang, Y.; Chen, D.; Shi, Z.; Smith, A.D.; Li, W.; Gao, Y. Central Nervous System Diseases Related to Pathological Microglial Phagocytosis. CNS Neurosci. Ther. 2021, 27, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Fiebich, B.L.; Ribeiro, C.; Batista, A.; Saliba, S.W.; Yousif, N.M.; Pinheiro De Oliveira, A.C.; Burguillos, M.A.; Airavaara, M.; Carlos, A.; De Oliveira, P. Role of Microglia TLRs in Neurodegeneration. Front. Cell. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 473145. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, L.A.; Di Gioia, M.; Amarante-Mendes, G.P.; Br, G.; Weinlich, R.; Bortoluci, K.R.; Adjemian, S.; Branco, L.M.; Zanetti, L.C. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Front. Immunol. 2018, 9, 2379. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Pablo, J.; Vaccari, R.; Dalton Dietrich, W.; Popovich, P.G.; Keane, R.W. Pattern Recognition Receptors and Central Nervous System Repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2015, 53, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Duan, L.; Xiong, H.; Jiang, Y.; Liang, H. Microglia Activation Mediated by Toll-like Receptor-4 Impairs Brain White Matter Tracts in Rats. J. Biomed. Res. 2018, 32, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; De Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Falsig, J.; Van Beek, J.; Hermann, C.; Leist, M. Review Molecular Basis for Detection of Invading Pathogens in the Brain. J. Neurosci. Res. 2007, 86, 1434–1447. [Google Scholar] [CrossRef]
- Hori, O.; Brett, J.; Slattery, T.; Cao, R.; Zhang, J.; Chen, J.X.; Nagashima, M.; Lundh, E.R.; Vijay, S.; Nitecki, D.; et al. The Receptor for Advanced Glycation End Products (RAGE) Is a Cellular Binding Site for Amphoterin. J. Biol. Chem. 1995, 270, 25752–25761. [Google Scholar] [CrossRef]
- Figdor, C.G.; van Kooyk, Y.; Adema Gosse, J. C-Type Lectin Receptors on Dendritic Cells and Langerhans Cells. Nat. Rev. Immunol. 2002, 2, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, H.; Prabagar, M.G.V.; Choi, W.S.; Kim, S.J.; Cheong, C.; Park, C.G.; Chin, C.Y.; Kang, Y.S. The C-Type Lectin CD209b Is Expressed on Microglia and It Mediates the Uptake of Capsular Polysaccharides of Streptococcus Pneumoniae. Neurosci. Lett. 2009, 450, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Chiu, I.; Wang, Y.; Sloane, J.A.; Lü, J.; Kosaras, B.; Sidman, R.L.; Volpe, J.J.; Vartanian, T. Toll-like Receptor 8 Functions as a Negative Regulator of Neurite Outgrowth and Inducer of Neuronal Apoptosis. J. Cell Biol. 2006, 175, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, I.E.; Lewen, A.; Galow, L.V.; Cesetti, T.; Scheffel, J.; Regen, T.; Hanisch, U.K.; Kann, O. TLR4-Activated Microglia Require IFN-γ to Induce Severe Neuronal Dysfunction and Death in Situ. Proc. Natl. Acad. Sci. USA 2016, 113, 212–217. [Google Scholar] [CrossRef]
- Ebert, S.; Gerber, J.; Bader, S.; Mühlhauser, F.; Brechtel, K.; Mitchell, T.J.; Nau, R. Dose-Dependent Activation of Microglial Cells by Toll-like Receptor Agonists Alone and in Combination. J. Neuroimmunol. 2005, 159, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.M.; Rodríguez, J.; Giambartolomei, G.H. Microglia at the Crossroads of Pathogen-Induced Neuroinflammation. ASN Neuro 2022, 14, 17590914221104566. [Google Scholar] [CrossRef] [PubMed]
- Machado-Pereira, M.; Santos, T.; Ferreira, L.; Bernardino, L.; Ferreira, R. Anti-Inflammatory Strategy for M2 Microglial Polarization Using Retinoic Acid-Loaded Nanoparticles. Mediat. Inflamm. 2017, 2017, 6742427. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, Z.; Jiang, X.; Qu, L.; Zhou, W.; Sun, X.; Hou, J.; Xu, H.; Cheng, M. Gut Microbiota Taxon-Dependent Transformation of Microglial M1/M2 Phenotypes Underlying Mechanisms of Spatial Learning and Memory Impairment after Chronic Methamphetamine Exposure. Microbiol. Spectr. 2023, 11, e0030223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Yang, T.; Oliveira, A.C.; Lobaton, G.O.; Aquino, V.; Kim, S.; Richards, E.M.; Pepine, C.J.; Sumners, C.; Raizada, M.K. Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension. Circ. Res. 2019, 124, 727–736. [Google Scholar] [CrossRef]
- Mossad, O.; Batut, B.; Yilmaz, B.; Dokalis, N.; Mezö, C.; Nent, E.; Nabavi, L.S.; Mayer, M.; Maron, F.J.M.; Buescher, J.M.; et al. Gut Microbiota Drives Age-Related Oxidative Stress and Mitochondrial Damage in Microglia via the Metabolite N 6-Carboxymethyllysine. Nat. Neurosci. 2022, 25, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Barres, B.A. A Smarter Mouse with Human Astrocytes. BioEssays 2013, 35, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, M.; Menard, F. Glutamate Signaling and Filopodiagenesis of Astrocytoma Cells in Brain Cancers: Survey and Questions. Cells 2022, 11, 2657. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-Synapse Structural Plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type i Interferons and Microbial Metabolites of Tryptophan Modulate Astrocyte Activity and Central Nervous System Inflammation via the Aryl Hydrocarbon Receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, M. Glutamate Induced Morphological Response in Astrocytoma Cells. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2021. [Google Scholar] [CrossRef]
- Tabatabaee, M.S.; Menard, F. L-Type Voltage-Gated Calcium Channel Modulators Inhibit Glutamate-Induced Morphology Changes in U118-MG Astrocytoma Cells. Cell Mol. Neurobiol. 2020, 40, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Willard, S.S.; Koochekpour, S. Glutamate, Glutamate Receptors, and Downstream Signaling Pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Malarkey, E.B.; Parpura, V. Mechanisms of Glutamate Release from Astrocytes. Neurochem. Int. 2008, 52, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.M.; Sun, M.F.; Jia, X.B.; Li, Y.; Zhang, B.P.; Zhao, L.P.; Shi, Y.; Zhou, Z.L.; Zhu, Y.L.; Cui, C.; et al. Sodium Butyrate Exacerbates Parkinson’s Disease by Aggravating Neuroinflammation and Colonic Inflammation in MPTP-Induced Mice Model. Neurochem. Res. 2020, 45, 2128–2142. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, J.; Suuronen, T.; Nuutinen, T.; Kyrylenko, S.; Salminen, A. Regulation of Microglial Inflammatory Response by Sodium Butyrate and Short-Chain Fatty Acids. Br. J. Pharmacol. 2004, 141, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Tan, V.; Heng, B.; Mohammadipanah, F.; Guillemin, G.J. Protective Effects of Myxobacterial Extracts on Hydrogen Peroxide-Induced Toxicity on Human Primary Astrocytes. Neuroscience 2019, 399, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cooley, I.D.; Chauhan, V.S.; Donneyz, M.A.; Marriott, I. Astrocytes Produce IL-19 in Response to Bacterial Challenge and Are Sensitive to the Immunosuppressive Effects of This IL-10 Family Member. Glia 2014, 62, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, A.R.; Johnson, M.B.; Yaemmongkol, J.J.; Marriott, I. Murine Astrocytes Produce IL-24 and Are Susceptible to the Immunosuppressive Effects of This Cytokine. J. Neuroinflamm. 2019, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Chang, M.-S. IL-20 Is Regulated by Hypoxia-Inducible Factor and up-Regulated after Experimental Ischemic Stroke. J. Immunol. 2009, 182, 5003–5012. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, T.; Wada, S.; Suzuki, S.; Okuma, Y.; Akira, S.; Matsuda, T.; Nomura, Y. Bacterial Endotoxin Induces IL-20 Expression in the Glial Cells. Mol. Brain Res. 2004, 130, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Heithoff, B.P.; George, K.K.; Phares, A.N.; Zuidhoek, I.A.; Munoz-Ballester, C.; Robel, S. Astrocytes Are Necessary for Blood–Brain Barrier Maintenance in the Adult Mouse Brain. Glia 2021, 69, 436–472. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–Endothelial Interactions at the Blood–Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Sterka, D.; Rati, D.M.; Marriott, I. Functional Expression of NOD2, a Novel Pattern Recognition Receptor for Bacterial Motifs, in Primary Murine Astrocytes. Glia 2006, 53, 322–330. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Wang, Z.; Zhang, X.; Yao, L.; Wang, F.; Liu, S.; Yin, J.; Ling, E.A.; Wang, L.; et al. High Glucose-Induced Expression of Inflammatory Cytokines and Reactive Oxygen Species in Cultured Astrocytes. Neuroscience 2012, 202, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Neuhaus, J.; Kniesel, U.; Krauß, B.; Schmid, E.M.; Öcalan, M.; Farrell, C.; Risau, W. Modulation of Tight Junction Structure in Blood-Brain Barrier Endothelial Cells Effects of Tissue Culture, Second Messengers and Cocultured Astrocytes. J. Cell Sci. 1994, 107, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Stachulski, A.V.; Knausenberger, T.B.A.; Shah, S.N.; Hoyles, L.; McArthur, S. A Host–Gut Microbial Amino Acid Co-Metabolite, p-Cresol Glucuronide, Promotes Blood–Brain Barrier Integrity in Vivo. Tissue Barriers 2023, 11, 2073175. [Google Scholar] [CrossRef] [PubMed]
- Ntranos, A.; Casaccia, P. The Microbiome–Gut–Behavior Axis: Crosstalk Between the Gut Microbiome and Oligodendrocytes Modulates Behavioral Responses. Neurotherapeutics 2018, 15, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.B.; Silva, B.A.; Perova, Z.; Marrone, L.; Masferrer, M.E.; Zhan, Y.; Kaplan, A.; Greetham, L.; Verrechia, V.; Halman, A.; et al. Prefrontal Cortical Control of a Brainstem Social Behavior Circuit. Nat. Neurosci. 2017, 20, 260. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of Prefrontal Cortex Myelination by the Microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef]
- Gacias, M.; Gaspari, S.; Santos, P.M.G.; Tamburini, S.; Andrade, M.; Zhang, F.; Shen, N.; Tolstikov, V.; Kiebish, M.A.; Dupree, J.L.; et al. Microbiota-Driven Transcriptional Changes in Prefrontal Cortex Override Genetic Differences in Social Behavior. Elife 2016, 5, e13442. [Google Scholar] [CrossRef] [PubMed]
- Myatich, A.; Haque, A.; Sole, C.; Banik, N.L. Clemastine in Remyelination and Protection of Neurons and Skeletal Muscle after Spinal Cord Injury. Neural Regen. Res. 2023, 18, 940. [Google Scholar] [CrossRef] [PubMed]
- Kalafatakis, I.; Karagogeos, D. Oligodendrocytes and Microglia: Key Players in Myelin Development, Damage and Repair. Biomolecules 2021, 11, 1058. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Waubant, E. The Multiple Sclerosis Microbiome? Ann. Transl. Med. 2017, 5, 12015. [Google Scholar] [CrossRef] [PubMed]
- Branton, W.G.; Lu, J.Q.; Surette, M.G.; Holt, R.A.; Lind, J.; Laman, J.D.; Power, C. Brain Microbiota Disruption within Inflammatory Demyelinating Lesions in Multiple Sclerosis. Sci. Rep. 2016, 6, 37344. [Google Scholar] [CrossRef] [PubMed]
- Glaum, S.R.; Holzwarth, J.A.; Miller, R.J. Glutamate Receptors Activate Ca2+ Mobilization and Ca2+ Influx into Astrocytes. Proc. Natl. Acad. Sci. USA 1990, 87, 3454–3458. [Google Scholar] [CrossRef]
- Backus, K.H.; Kettenmann, H.; Schachner, M. Pharmacological Characterization of the Glutamate Receptor in Cultured Astrocytes. J. Neurosci. Res. 1989, 22, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.R.; Koji Takahashi, D.; Thomson, K.E.; Wilcox, K.S. The Expression of Kainate Receptor Subunits in Hippocampal Astrocytes After Experimentally Induced Status Epilepticus. J. Neuropathol. Exp. Neurol. 2013, 72, 919–932. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.P.; Fox, B.W.; Chao, P.H.; Schroeder, F.C.; Sengupta, P. A Neurotransmitter Produced by Gut Bacteria Modulates Host Sensory Behaviour. Nature 2020, 583, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.F.; Verkhratsky, A.; Parpura, V. Caenorhabditis Elegans Glia Modulate Neuronal Activity and Behavior. Front. Cell Neurosci. 2014, 8, 81214. [Google Scholar] [CrossRef]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Dooling, S.W.; Costa-Mattioli, M. Gut Bacteria Seize Control of the Brain to Prevent Epilepsy. Cell Host Microbe 2018, 24, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Castro, A.J.; Gonzalez-Cervantes, R.M.; Bravo-Ruiseco, G.; Pacheco-Lopez, G. The Microbiota-Gut-Brain Axis: Neurobehavioral Correlates, Health and Sociality. Front. Integr. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Aceto, S.; Agnisola, C.; De Paolo, S.; Dipineto, L.; Stilling, R.M.; Dinan, T.G.; Cryan, J.F.; Menna, L.F.; Fioretti, A. Probiotic Modulation of the Microbiota-Gut-Brain Axis and Behaviour in Zebrafish. Sci. Rep. 2016, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Di Meco, A.; Dodiya, H.B.; Popovic, J.; Cuddy, L.K.; Weigle, I.Q.; Zhang, X.; Sadleir, K.; Sisodia, S.S.; Vassar, R. The Gut Microbiome Regulates Astrocyte Reaction to Aβ Amyloidosis through Microglial Dependent and Independent Mechanisms. Mol. Neurodegener. 2023, 18, 45. [Google Scholar] [CrossRef]
- Bulgart, H.R.; Neczypor, E.W.; Wold, L.E.; Mackos, A.R. Microbial Involvement in Alzheimer Disease Development and Progression. Mol. Neurodegener. 2020, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.; Gruenheid, S. Microbes and Parkinson’s Disease: From Associations to Mechanisms. Trends Microbiol. 2022, 30, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Hickman, R.A.; Faustin, A.; Wisniewski, T. Alzheimer Disease and Its Growing Epidemic: Risk Factors, Biomarkers, and the Urgent Need for Therapeutics. Neurol. Clin. 2016, 34, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Sabatini, B.L.; Südhof, T.C. Synapses and Alzheimer’s Disease. Cold Spring Harb. Perspect. Biol. 2012, 4, a005777. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Nunes-Costa, D.; Magalhães, J.D.; G-Fernandes, M.; Cardoso, S.M.; Empadinhas, N. Microbial BMAA and the Pathway for Parkinson’s Disease Neurodegeneration. Front. Aging Neurosci. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Erin, R.; Reyes, N.; Gao, L.; Asatryan, L. Neuroimmunology and Neuroinflammation Microbiome Meets Microglia in Neuroinflammation and Neurological Disorders. Neurosciences 2020, 7, 215–233. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabatabaee, M. Microbiome-Glia Crosstalk: Bridging the Communication Divide in the Central Nervous System. Neuroglia 2024, 5, 89-104. https://doi.org/10.3390/neuroglia5020007
Tabatabaee M. Microbiome-Glia Crosstalk: Bridging the Communication Divide in the Central Nervous System. Neuroglia. 2024; 5(2):89-104. https://doi.org/10.3390/neuroglia5020007
Chicago/Turabian StyleTabatabaee, Mitra. 2024. "Microbiome-Glia Crosstalk: Bridging the Communication Divide in the Central Nervous System" Neuroglia 5, no. 2: 89-104. https://doi.org/10.3390/neuroglia5020007
APA StyleTabatabaee, M. (2024). Microbiome-Glia Crosstalk: Bridging the Communication Divide in the Central Nervous System. Neuroglia, 5(2), 89-104. https://doi.org/10.3390/neuroglia5020007