Six Decades of Dopamine Hypothesis: Is Aryl Hydrocarbon Receptor the New D2?
Abstract
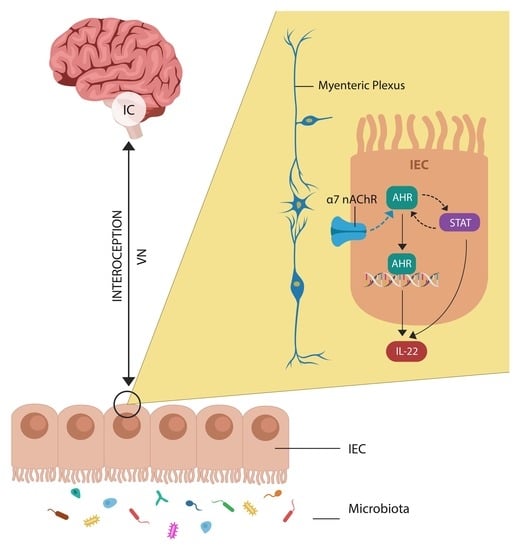
:1. Introduction
2. DH and SCZ Outcome Studies
- 1901–1920, 20% complete recovery, 4.7% employed;
- 1921–1940, 12% complete recovery, 11.9% employed;
- 1941–1955, 23% complete recovery, 4.1% employed;
- 1956–1975, 20% complete recovery, 5.1% employed;
- 1976–1995, 20% complete recovery, 6.9% employed.
- Continued need for long-term public institutions for the treatment of chronic mental illness.
- DA blockers may not alter the progression of SCZ toward disability and cognitive deficit.
- Several SCZ characteristics are difficult to reconcile with the DH.
- Life-long gray matter loss occurs despite treatment with DA blockers.
- Upregulated DA in the CNS would be expected to result in euphoria, increased motivation, and alertness rather than hallucinations or delusions.
- Anosognosia, the most common symptom of SCZ, is infrequently influenced by DA-blocking treatments.
3. Insight vs. Anosognosia, Lessons from COVID-19 and HIV
3.1. Mononuclear Cells and Insight
3.2. Cholinergic Anti-Inflammatory Pathway and Insight
4. DH-Incongruent SCZ Features
4.1. Markers of Gut Barrier Dysfunction
4.2. Autoantibodies as Markers of Microbial Translocation
4.3. AhR, and Antipsychotic Drugs
4.4. AhR and Environmental Pollutants
4.5. AhR and Latitude Variance
5. Non-Dopaminergic Antipsychotic Mechanisms of Neuroleptic Drugs
5.1. Antipsychotics as Antibacterials
5.2. Antipsychotics as Antivirals
5.3. Antipsychotics as Anticancer Drugs
5.4. Microbial Phenazines vs. Antipsychotic Phenothiazines
6. Potential Applications
6.1. Cholinergic Anti-Inflammatory Pathway Augmentation
6.2. Recombinant IL-22 for IBD and SCZ
6.3. Dietary and Pharmacological AhR Ligands
7. Wider Disparate Data and Future Directions
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumeister, A.A. The Chlorpromazine Enigma. J. Hist. Neurosci. 2013, 22, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A. Basic concepts underlying recent developments in the field of Parkinson’s disease. Contemp. Neurol. Ser. 1971, 8, 1–31. [Google Scholar] [PubMed]
- McKenna, P.J. Pathology, Phenomenology and the Dopamine Hypothesis of Schizophrenia. Br. J. Psychiatry 1987, 151, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.H. The dopamine hypothesis of schizophrenia: Focus on the dopamine receptor. Am. J. Psychiatry 1976, 133, 197–202. [Google Scholar] [CrossRef]
- Nehme, H.; Saulnier, P.; Ramadan, A.A.; Cassisa, V.; Guillet, C.; Eveillard, M.; Umerska, A. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS ONE 2018, 13, e0189950. [Google Scholar] [CrossRef]
- Hirata, Y.; Oka, K.; Yamamoto, S.; Watanabe, H.; Oh-Hashi, K.; Hirayama, T.; Nagasawa, H.; Takemori, H.; Furuta, K. Haloperidol Prevents Oxytosis/Ferroptosis by Targeting Lysosomal Ferrous Ions in a Manner Independent of Dopamine D2 and Sigma-1 Receptors. ACS Chem. Neurosci. 2022, 13, 2719–2727. [Google Scholar] [CrossRef]
- Iasevoli, F.; Avagliano, C.; D’ambrosio, L.; Barone, A.; Ciccarelli, M.; De Simone, G.; Mazza, B.; Vellucci, L.; de Bartolomeis, A. Dopamine Dynamics and Neurobiology of Non-Response to Antipsychotics, Relevance for Treatment Resistant Schizophrenia: A Systematic Review and Critical Appraisal. Biomedicines 2023, 11, 895. [Google Scholar] [CrossRef]
- Yang, A.C.; Tsai, S.-J. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int. J. Mol. Sci. 2017, 18, 1689. [Google Scholar] [CrossRef] [Green Version]
- Woldman, I.; Reither, H.; Kattinger, A.; Hornykiewicz, O.; Pifl, C. Dopamine inhibits cell growth and cell cycle by blocking ribonucleotide reductase. Neuropharmacology 2005, 48, 525–537. [Google Scholar] [CrossRef]
- Papadopoulos, F.; Isihou, R.; Alexiou, G.A.; Tsalios, T.; Vartholomatos, E.; Markopoulos, G.S.; Sioka, C.; Tsekeris, P.; Kyritsis, A.P.; Galani, V. Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells. Biomedicines 2020, 8, 595. [Google Scholar] [CrossRef]
- Bernstein, C.N.; A Hitchon, C.; Walld, R.; Bolton, J.M.; Sareen, J.; Walker, J.R.; A Graff, L.; Patten, S.B.; Singer, A.; Lix, L.M.; et al. Increased Burden of Psychiatric Disorders in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 25, 360–368. [Google Scholar] [CrossRef]
- Maes, M.; Kanchanatawan, B.; Sirivichayakul, S.; Carvalho, A.F. In Schizophrenia, Increased Plasma IgM/IgA Responses to Gut Commensal Bacteria Are Associated with Negative Symptoms, Neurocognitive Impairments, and the Deficit Phenotype. Neurotox. Res. 2018, 35, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Secher, T.; Samba-Louaka, A.; Oswald, E.; Nougayrède, J.-P. Escherichia coli Producing Colibactin Triggers Premature and Transmissible Senescence in Mammalian Cells. PLoS ONE 2013, 8, e77157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Gao, F.; Zhou, L.; Fan, Y.; Zhao, B.; Xi, W.; Wang, C.; Zhu, F.; Ma, X.; Wang, W.; et al. Characterizing serum amino acids in schizophrenic patients: Correlations with gut microbes. J. Psychiatr. Res. 2022, 153, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Zhang, B.; Wang, H.E.; Bai, Y.; Tsai, S.; Su, T.; Chen, T.; Hou, M.; Lu, C.; Wang, Y.; et al. Schizophrenia and risk of new-onset inflammatory bowel disease: A nationwide longitudinal study. Aliment. Pharmacol. Ther. 2022, 55, 1192–1201. [Google Scholar] [CrossRef]
- Bartocci, B.; Buono, A.D.; Gabbiadini, R.; Busacca, A.; Quadarella, A.; Repici, A.; Mencaglia, E.; Gasparini, L.; Armuzzi, A. Mental Illnesses in Inflammatory Bowel Diseases: Mens sana in corpore sano. Medicina 2023, 59, 682. [Google Scholar] [CrossRef]
- Helm, E.Y.; Zhou, L. Transcriptional regulation of innate lymphoid cells and T cells by aryl hydrocarbon receptor. Front Immunol. 2023, 14, 1056267. [Google Scholar] [CrossRef]
- Sewell, D.D. Schizophrenia and HIV. Schizophr. Bull. 1996, 22, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, D.S.; Lorenz, J. Anosognosia in schizophrenia: Hidden in plain sight. Innov. Clin. Neurosci. 2014, 11, 10–17. [Google Scholar]
- Torregrossa, L.J.; Amedy, A.; Roig, J.; Prada, A.; Park, S. Interoceptive functioning in schizophrenia and schizotypy. Schizophr. Res. 2021, 239, 151–159. [Google Scholar] [CrossRef]
- Ardizzi, M.; Ambrosecchia, M.; Buratta, L.; Ferri, F.; Peciccia, M.; Donnari, S.; Mazzeschi, C.; Gallese, V. Interoception and Positive Symptoms in Schizophrenia. Front. Hum. Neurosci. 2016, 10, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, T.A.; Ullsperger, M.; Danielmeier, C. Error awareness and the insula: Links to neurological and psychiatric diseases. Front. Hum. Neurosci. 2013, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Lievana, E.; Ramírez-Mejía, G.; Urrego-Morales, O.; Luis-Islas, J.; Gutierrez, R.; Bermúdez-Rattoni, F. Photostimulation of Ventral Tegmental Area-Insular Cortex Dopaminergic Inputs Enhances the Salience to Consolidate Aversive Taste Recognition Memory via D1-Like Receptors. Front. Cell. Neurosci. 2022, 16, 823220. [Google Scholar] [CrossRef] [PubMed]
- Karnath, H.-O.; Baier, B.; Nägele, T. Awareness of the Functioning of One’s Own Limbs Mediated by the Insular Cortex? J. Neurosci. 2005, 25, 7134–7138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullsperger, M.; Harsay, H.A.; Wessel, J.R.; Ridderinkhof, K.R. Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct. 2010, 214, 629–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Martín, F.J.; Fernández-Salguero, P.M.; Merino, J.M. Aryl hydrocarbon receptor-dependent induction of apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in cerebellar granule cells from mouse. J. Neurochem. 2011, 118, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Attademo, L.; Bernardini, F. Air Pollution as Risk Factor for Mental Disorders: In Search for a Possible Link with Alzheimer’s Disease and Schizophrenia. J. Alzheimer Dis. 2020, 76, 825–830. [Google Scholar] [CrossRef]
- Antonsen, S.; Mok, P.L.H.; Webb, R.T.; Mortensen, P.B.; McGrath, J.J.; Agerbo, E.; Brandt, J.; Geels, C.; Christensen, J.H.; Pedersen, C.B. Exposure to air pollution during childhood and risk of developing schizophrenia: A national cohort study. Lancet Planet. Health 2020, 4, e64–e73. [Google Scholar] [CrossRef] [Green Version]
- Dagher, J.B.; Al Mansi, M.; Jacob, E.; Kaimal, A.; Chuang, Y.; Mohankumar, P.S.; MohanKumar, S.M.J. Prenatal Exposure to Bisphenol A and Diethylhexyl Phthalate Induces Apoptosis in the Thymus of Male and Female Offspring. FASEB J. 2019, 33, 812.5. [Google Scholar] [CrossRef]
- Maurya, P.K.; Rizzo, L.B.; Xavier, G.; Tempaku, P.F.; Ota, V.K.; Santoro, M.L.; Spíndola, L.M.; Moretti, P.S.; Mazzotti, D.R.; Gadelha, A.; et al. Leukocyte telomere length variation in different stages of schizophrenia. J. Psychiatr. Res. 2018, 96, 218–223. [Google Scholar] [CrossRef]
- Holahan, M.R.; Smith, C.A.; Luu, B.E.; Storey, K.B. Preadolescent Phthalate (DEHP) Exposure Is Associated With Elevated Locomotor Activity and Reward-Related Behavior and a Reduced Number of Tyrosine Hydroxylase Positive Neurons in Post-Adolescent Male and Female Rats. Toxicol. Sci. 2018, 165, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Menon, R.; Manteiga, S.; Alden, N.; Hunt, C.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Environmental Chemical Diethylhexyl Phthalate Alters Intestinal Microbiota Community Structure and Metabolite Profile in Mice. Msystems 2019, 4, e00724-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Kim, W.-H.; Kim, Y.-Y.; Park, H.-Y. Air Pollution and Central Nervous System Disease: A Review of the Impact of Fine Particulate Matter on Neurological Disorders. Front. Public Health 2020, 8, 575330. [Google Scholar] [CrossRef]
- Dey, S.K.; Sugur, K.; Venkatareddy, V.G.; Rajeev, P.; Gupta, T.; Thimmulappa, R.K. Lipid peroxidation index of particulate matter: Novel metric for quantifying intrinsic oxidative potential and predicting toxic responses. Redox Biol. 2021, 48, 102189. [Google Scholar] [CrossRef]
- Li, X.; Luck, M.E.; Hammer, A.M.; Cannon, A.R.; Choudhry, M.A. 6-Formylindolo (3, 2-b) Carbazole (FICZ)–mediated protection of gut barrier is dependent on T cells in a mouse model of alcohol combined with burn injury. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165901. [Google Scholar] [CrossRef] [PubMed]
- Memari, B.; Nguyen-Yamamoto, L.; Salehi-Tabar, R.; Zago, M.; Fritz, J.H.; Baglole, C.J.; Goltzman, D.; White, J.H. Endocrine aryl hydrocarbon receptor signaling is induced by moderate cutaneous exposure to ultraviolet light. Sci. Rep. 2019, 9, 8486. [Google Scholar] [CrossRef] [Green Version]
- Rannug, A.; Fritsche, E. The aryl hydrocarbon receptor and light. Biol. Chem. 2006, 387, 1149–1157. [Google Scholar] [CrossRef]
- Saatci, D.; Johnson, T.; Smee, M.; van Nieuwenhuizen, A.; Handunnetthi, L. The role of latitude and infections in the month-of-birth effect linked to schizophrenia. Brain Behav. Immun. Health 2022, 24, 100486. [Google Scholar] [CrossRef]
- Dikongué, E.; Ségurel, L. Latitude as a co-driver of human gut microbial diversity? Bioessays 2017, 39, 1600145. [Google Scholar] [CrossRef]
- Krøll, J. E. coli antibodies in schizophrenia. Psychol. Med. 1986, 16, 209–211. [Google Scholar] [CrossRef]
- Al-Diwani, A.A.J.; Pollak, T.A.; Irani, S.R.; Lennox, B.R. Psychosis: An autoimmune disease? Immunology 2017, 152, 388–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagorn, A.; Chapalain, A.; Mijouin, L.; Hillion, M.; Duclairoir-Poc, C.; Chevalier, S.; Taupin, L.; Orange, N.; Feuilloley, M.G.J. Effect of GABA, a Bacterial Metabolite, on Pseudomonas fluorescens Surface Properties and Cytotoxicity. Int. J. Mol. Sci. 2013, 14, 12186–12204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, A.; Abe, H.; Tsuruta, S.; Chiba, S.; Fujii-Kuriyama, Y.; Sekiya, T.; Morita, R.; Yoshimura, A. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int. Immunol. 2013, 26, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postal, B.G.; Ghezzal, S.; Aguanno, D.; André, S.; Garbin, K.; Genser, L.; Brot-Laroche, E.; Poitou, C.; Soula, H.; Leturque, A.; et al. AhR activation defends gut barrier integrity against damage occurring in obesity. Mol. Metab. 2020, 39, 101007. [Google Scholar] [CrossRef]
- Ishima, T.; Iyo, M.; Hashimoto, K. Neurite outgrowth mediated by the heat shock protein Hsp90α: A novel target for the antipsychotic drug aripiprazole. Transl. Psychiatry 2012, 2, e170. [Google Scholar] [CrossRef] [Green Version]
- McFarland, N.R.; Dimant, H.; Kibuuka, L.; Ebrahimi-Fakhari, D.; Desjardins, C.A.; Danzer, K.M.; Danzer, M.; Fan, Z.; Schwarzschild, M.A.; Hirst, W.; et al. Chronic Treatment with Novel Small Molecule Hsp90 Inhibitors Rescues Striatal Dopamine Levels but Not α-Synuclein-Induced Neuronal Cell Loss. PLoS ONE 2014, 9, e86048. [Google Scholar] [CrossRef]
- Carver, L.; Jackiw, V.; Bradfield, C. The 90-kDa heat shock protein is essential for Ah receptor signaling in a yeast expression system. J. Biol. Chem. 1994, 269, 30109–30112. [Google Scholar] [CrossRef]
- Whitelaw, M.L.; McGuire, J.; Picard, D.; A Gustafsson, J.; Poellinger, L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 4437–4441. [Google Scholar] [CrossRef]
- Kaneta, H.; Ukai, W.; Tsujino, H.; Furuse, K.; Kigawa, Y.; Tayama, M.; Ishii, T.; Hashimoto, E.; Kawanishi, C. Antipsychotics promote GABAergic interneuron genesis in the adult rat brain: Role of heat-shock protein production. J. Psychiatr. Res. 2017, 92, 108–118. [Google Scholar] [CrossRef]
- Uemura, S.; Nakajima, Y.; Yoshida, Y.; Furuya, M.; Matsutani, S.; Kawate, S.; Ikeda, S.-I.; Tsuji, N.; Grave, E.; Wakui, H.; et al. Biochemical properties of human full-length aryl hydrocarbon receptor (AhR). J. Biochem. 2020, 168, 285–294. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, S.J.; Toh, K.Y.; Lee, C.U.; Lee, C.; Paik, I.H. Identification of antibodies to heat shock proteins 90 kDa and 70 kDa in patients with schizophrenia. Schizophr. Res. 2001, 52, 127–135. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, W.; Liu, Y.; Zhang, J.; Lu, Y.; Wan, X.; Qiao, Y.; Huang, H.; Zeng, Z.; Li, W.; et al. Extracellular HSP90α promotes cellular senescence by modulating TGF -β signaling in pulmonary fibrosis. FASEB J. 2022, 36, e22475. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jin, U.-H.; Karki, K.; Jayaraman, A.; Allred, C.H.; Michelhaugh, S.K.; Mittal, S.; Chapkin, R.S.; Safe, S.H. Dopamine is an aryl hydrocarbon receptor agonist. Biochem. J. 2020, 477, 3899–3910. [Google Scholar] [CrossRef]
- Fehsel, K.; Schwanke, K.; Kappel, B.; Fahimi, E.; Meisenzahl-Lechner, E.; Esser, C.; Hemmrich, K.; Haarmann-Stemmann, T.; Kojda, G.; Lange-Asschenfeldt, C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J. Psychopharmacol. 2022, 36, 191–201. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Åneman, A.; Friberg, P.; Hooper, D.; Fåndriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial Production of Dopamine in the Human Gastrointestinal Tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, A.; Mackie, P.M.; Phan, L.T.; Mirabel, R.; Smith, A.R.; Miller, E.; Franks, S.; Syed, O.; Riaz, T.; Law, B.K.; et al. Who Knew? Dopamine Transporter Activity Is Critical in Innate and Adaptive Immune Responses. Cells 2023, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, P.; Tian, H.; Tian, F.; Zhang, Y.; Zhang, L.; Gao, X.; Wang, X. Aryl hydrocarbon receptor/IL-22/Stat3 signaling pathway is involved in the modulation of intestinal mucosa antimicrobial molecules by commensal microbiota in mice. Innate Immun. 2018, 24, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Le, P.T.; Pearce, M.M.; Zhang, S.; Campbell, E.M.; Fok, C.S.; Mueller, E.R.; Brincat, C.A.; Wolfe, A.J.; Brubaker, L. IL22 Regulates Human Urothelial Cell Sensory and Innate Functions through Modulation of the Acetylcholine Response, Immunoregulatory Cytokines and Antimicrobial Peptides: Assessment of an In Vitro Model. PLoS ONE 2014, 9, e111375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoda, H. Role of nicotinic acetylcholine receptors for modulation of microcircuits in the agranular insular cortex. J. Oral Biosci. 2018, 61, 5–11. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, K.; Han, B.; Sheng, B.; Yin, J.; Pu, A.; Li, L.; Sun, L.; Yu, M.; Qiu, Y.; et al. Aryl hydrocarbon receptor inhibits inflammation in DSS-induced colitis via the MK2/p-MK2/TTP pathway. Int. J. Mol. Med. 2017, 41, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.; Cheng, W.; Zheng, T.; Jiang, N.; Xu, R. AHR is a tunable knob that controls HTLV-1 latency-reactivation switching. PLoS Pathog. 2020, 16, e1008664. [Google Scholar] [CrossRef]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Huang, A.S.; Rogers, B.P.; Blackford, J.U.; Heckers, S.; Woodward, N.D. Insula sub-regions across the psychosis spectrum: Morphology and clinical correlates. Transl. Psychiatry 2021, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Yawata, Y.; Shikano, Y.; Ogasawara, J.; Makino, K.; Kashima, T.; Ihara, K.; Yoshimoto, A.; Morikawa, S.; Yagishita, S.; Tanaka, K.F.; et al. Mesolimbic dopamine release precedes actively sought aversive stimuli in mice. Nat. Commun. 2023, 14, 2433. [Google Scholar] [CrossRef] [PubMed]
- Coffeen, U.; López-Avila, A.; Ortega-Legaspi, J.M.; Ángel, R.; López-Muñoz, F.J.; Pellicer, F. Dopamine receptors in the anterior insular cortex modulate long-term nociception in the rat. Eur. J. Pain 2008, 12, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Loohuis, L.M.O.; Mangul, S.; Ori, A.P.S.; Jospin, G.; Koslicki, D.; Yang, H.T.; Wu, T.; Boks, M.P.; Lomen-Hoerth, C.; Wiedau-Pazos, M.; et al. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl. Psychiatry 2018, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Alekseyenko, A.V.; Ogunrinde, E.; Li, M.; Li, Q.-Z.; Huang, L.; Tsao, B.P.; Kamen, D.L.; Oates, J.C.; Li, Z.; et al. Rigorous Plasma Microbiome Analysis Method Enables Disease Association Discovery in Clinic. Front. Microbiol. 2021, 11, 613268. [Google Scholar] [CrossRef]
- Goren, I.; Brom, A.; Yanai, H.; Dagan, A.; Segal, G.; Israel, A. Risk of bacteremia in hospitalised patients with inflammatory bowel disease: A 9-year cohort study. United Eur. Gastroenterol. J. 2020, 8, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Kamat, A.; Ancuta, P.; Blumberg, R.S.; Gabuzda, D. Serological Markers for Inflammatory Bowel Disease in AIDS Patients with Evidence of Microbial Translocation. PLoS ONE 2010, 5, e15533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Origoni, A.E.; Khushalani, S.; Leweke, F.M.; Dickerson, F.B.; Yolken, R.H. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr. Res. 2013, 148, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Isnard, S.; Lin, J.; Fombuena, B.; Chatterjee, D.; Salinas, T.R.W.; Planas, D.; Cattin, A.; Fert, A.; Gabriel, E.M.; et al. Daily variations of gut microbial translocation markers in ART-treated HIV-infected people. AIDS Res. Ther. 2020, 17, 15. [Google Scholar] [CrossRef]
- Wallis, Z.K.; Williams, K.C. Monocytes in HIV and SIV Infection and Aging: Implications for Inflamm-Aging and Accelerated Aging. Viruses 2022, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Cherfane, C.; Click, B.; Ramos-Rivers, C.; E Koutroubakis, I.; Hashash, J.G.; Babichenko, D.; Tang, G.; Dunn, M.; Barrie, A.; et al. Monocytosis Is a Biomarker of Severity in Inflammatory Bowel Disease: Analysis of a 6-Year Prospective Natural History Registry. Inflamm. Bowel Dis. 2021, 28, 70–78. [Google Scholar] [CrossRef]
- Mazza, M.G.; Capellazzi, M.; Lucchi, S.; Tagliabue, I.; Rossetti, A.; Clerici, M. Monocyte count in schizophrenia and related disorders: A systematic review and meta-analysis. Acta Neuropsychiatr. 2020, 32, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ndirangu, J.; Viljoen, J.; Bland, R.M.; Danaviah, S.; Thorne, C.; Van de Perre, P.; Newell, M.-L. Cell-Free (RNA) and Cell-Associated (DNA) HIV-1 and Postnatal Transmission through Breastfeeding. PLoS ONE 2012, 7, e51493. [Google Scholar] [CrossRef] [Green Version]
- Vrablicova, Z.; Tomova, K.; Tothova, L.; Babickova, J.; Gromova, B.; Konecna, B.; Liptak, R.; Hlavaty, T.; Gardlik, R. Nuclear and Mitochondrial Circulating Cell-Free DNA Is Increased in Patients With Inflammatory Bowel Disease in Clinical Remission. Front. Med. 2020, 7, 593316. [Google Scholar] [CrossRef]
- Melamud, M.M.; Buneva, V.N.; Ermakov, E.A. Circulating Cell-Free DNA Levels in Psychiatric Diseases: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 3402. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Roo-Brand, G.; Lisotto, P.; Sadabad, M.S.; Reitsema, R.D.; de Goffau, M.C.; Faber, K.N.; Dijkstra, G.; Harmsen, H.J.M. Patients with Inflammatory Bowel Disease Show IgG Immune Responses Towards Specific Intestinal Bacterial Genera. Front. Immunol. 2022, 13, 842911. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, M.; Wu, Y.; Meng, Z.; Martin, L.; Zhang, L.; Ogunrinde, E.; Zhou, Z.; Qin, S.; Wan, Z.; et al. Systemic translocation of Staphylococcus drives autoantibody production in HIV disease. Microbiome 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Walter, M.; Glanz, W.; Sarnyai, Z.; Bernstein, H.-G.; Vielhaber, S.; Kästner, A.; Skalej, M.; Jordan, W.; Schiltz, K.; et al. Increased Prevalence of Diverse N -Methyl-D-Aspartate Glutamate Receptor Antibodies in Patients With an Initial Diagnosis of Schizophrenia: Specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry 2013, 70, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, Y.; Hashimoto, K.-I.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium glutamicum mechanosensitive channels: Towards unpuzzling “glutamate efflux” for amino acid production. Biophys. Rev. 2018, 10, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Klein, C.; Anton, J.J.; Kozlakidis, Z.; Andronescu, C.V. The Role of Lactylation in Mental Illness: Emphasis on Microglia. Neuroglia 2023, 4, 119–140. [Google Scholar] [CrossRef]
- Osorio, C.; Sfera, A.; Anton, J.J.; Thomas, K.G.; Andronescu, C.V.; Li, E.; Yahia, R.W.; Avalos, A.G.; Kozlakidis, Z. Virus-Induced Membrane Fusion in Neurodegenerative Disorders. Front. Cell. Infect. Microbiol. 2022, 12, 845580. [Google Scholar] [CrossRef]
- Davidson, L.; Schmutte, T.; Dinzeo, T.; Andres-Hyman, R. Remission and Recovery in Schizophrenia: Practitioner and Patient Perspectives. Schizophr. Bull. 2007, 34, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Leucht, S.; Lasser, R. The Concepts of Remission and Recovery in Schizophrenia. Pharmacopsychiatry 2006, 39, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Liberman, R.P.; Kopelowicz, A.; Ventura, J.; Gutkind, D. Operational criteria and factors related to recovery from schizophrenia. Int. Rev. Psychiatry 2002, 14, 256–272. [Google Scholar] [CrossRef]
- Silva, M.A.; Restrepo, D. Recuperación funcional en la esquizofrenia. Rev. Colomb. Psiquiatr. 2019, 48, 252–260. [Google Scholar] [CrossRef]
- Mathew, S.T.; Nirmala, B.P.; Kommu, J.V.S. Personal meaning of recovery among persons with schizophrenia. Int. J. Soc. Psychiatry 2021, 69, 78–85. [Google Scholar] [CrossRef]
- Ponce-Correa, F.; Caqueo-Urízar, A.; Berrios, R.; Escobar-Soler, C. Defining recovery in schizophrenia: A review of outcome studies. Psychiatry Res. 2023, 322, 115134. [Google Scholar] [CrossRef]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Yeomans, D.; Taylor, M.; Currie, A.; Whale, R.; Ford, K.; Fear, C.; Hynes, J.; Sullivan, G.; Moore, B.; Burns, T. Resolution and remission in schizophrenia: Getting well and staying well. Adv. Psychiatr. Treat. 2010, 16, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Becker, D.R.; Drake, R.E.; Bond, G.R.; Xie, H.; Dain, B.J.; Harrison, K. Job Terminations Among Persons with Severe Mental Illness Participating in Supported Employment. Community Ment. Health J. 1998, 34, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Bellack, A.S. Scientific and Consumer Models of Recovery in Schizophrenia. Schizophr. Bull. 2005, 32, 432–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zipursky, R.B. Why Are the Outcomes in Patients with Schizophrenia So Poor? J. Clin. Psychiatry 2014, 75, 20–24. [Google Scholar] [CrossRef]
- Üçok, A.; Polat, A.; Çakır, S.; Genç, A. One year outcome in first episode schizophrenia: Predictors of relapse. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 256, 37–43. [Google Scholar] [CrossRef]
- Holm, M.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Mitterdorfer-Rutz, E. Employment among people with schizophrenia or bipolar disorder: A population-based study using nationwide registers. Acta Psychiatr. Scand. 2020, 143, 61–71. [Google Scholar] [CrossRef]
- Lévesque, I.S.; Abdel-Baki, A. Homeless youth with first-episode psychosis: A 2-year outcome study. Schizophr. Res. 2019, 216, 460–469. [Google Scholar] [CrossRef]
- Harrison, G.; Hopper, K.; Craig, T.; Laska, E.; Siegel, C.; Wanderling, J.; Dube, K.C.; Ganev, K.; Giel, R.; Der Heiden, W.A.; et al. Recovery from psychotic illness: A 15- and 25-year international follow-up study. Br. J. Psychiatry 2001, 178, 506–517. [Google Scholar] [CrossRef] [Green Version]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A Systematic Review and Meta-Analysis of Recovery in Schizophrenia. Schizophr. Bull. 2012, 39, 1296–1306. [Google Scholar] [CrossRef] [Green Version]
- Kotov, R.; Fochtmann, L.; Li, K.; Tanenberg-Karant, M.; Constantino, E.A.; Rubinstein, J.; Perlman, G.; Velthorst, E.; Fett, A.-K.J.; Carlson, G.; et al. One hundred years of schizophrenia: A meta-analysis of the outcome literature. Am. J. Psychiatry 1994, 151, 1409–1416. [Google Scholar] [CrossRef]
- Warner, R. Recovery from Schizophrenia Psychiatry and Political Economy, 3rd ed.; Brunner-Routledge: Hove, UK; New York, NY, USA, 1997; p. 74. [Google Scholar]
- Mitelman, S.A.; Buchsbaum, M.S. Very poor outcome schizophrenia: Clinical and neuroimaging aspects. Int. Rev. Psychiatry 2007, 19, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Tsuang, M.T. Long-term Outcome of Major Psychoses: I. Schizophrenia and Affective Disorders Compared With Psychiatrically Symptom-Free Surgical Conditions. Arch. Gen. Psychiatry 1979, 36, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, S.; Johnson, S. Schizophrenia and employment. Soc. Psychiatry 2004, 39, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kim, H.; Wada, K.; Aboumrad, M.; Powell, E.; Zwain, G.; Benson, C.; Near, A.M. Unemployment, homelessness, and other societal outcomes in patients with schizophrenia: A real-world retrospective cohort study of the United States Veterans Health Administration database. BMC Psychiatry 2022, 22, 458. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Smieskova, R.; Kempton, M.; Ho, B.; Andreasen, N.; Borgwardt, S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013, 37, 1680–1691. [Google Scholar] [CrossRef] [Green Version]
- Ho, B.C.; Andreasen, N.C.; Ziebell, S.; Pierson, R.; Magnotta, V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 2011, 68, 128–137. [Google Scholar] [CrossRef] [Green Version]
- Cahn, W.; Pol HE, H.; Lems, E.B.; van Haren, N.E.; Schnack, H.G.; van der Linden, J.A.; Schothorst, P.F.; van Engeland, H.; Kahn, R.S. Brain volume changes in first-episode schizophrenia: A 1-year follow-up study. Arch. Gen. Psychiatry 2002, 59, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Howes, O.D.; Cummings, C.; Chapman, G.E.; Shatalina, E. Neuroimaging in schizophrenia: An overview of findings and their implications for synaptic changes. Neuropsychopharmacology 2022, 48, 151–167. [Google Scholar] [CrossRef]
- Leung, M.; Cheung, C.; Yu, K.; Yip, B.; Sham, P.; Li, Q.; Chua, S.; McAlonan, G. Gray Matter in First-Episode Schizophrenia Before and After Antipsychotic Drug Treatment. Anatomical Likelihood Estimation Meta-analyses With Sample Size Weighting. Schizophr. Bull. 2009, 37, 199–211. [Google Scholar] [CrossRef]
- Bodnar, M.; Malla, A.K.; Makowski, C.; Chakravarty, M.M.; Joober, R.; Lepage, M. The effect of second-generation antipsychotics on hippocampal volume in first episode of psychosis: Longitudinal study. BJPsych Open 2016, 2, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Vita, A.; De Peri, L.; Deste, G.; Barlati, S.; Sacchetti, E. The Effect of Antipsychotic Treatment on Cortical Gray Matter Changes in Schizophrenia: Does the Class Matter? A Meta-analysis and Meta-regression of Longitudinal Magnetic Resonance Imaging Studies. Biol. Psychiatry 2015, 78, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S. Kraepelin and the differential diagnosis of dementia praecox and manic-depressive insanity. Compr. Psychiatry 1986, 27, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Elghozi, J.-L.; Saad, M.A.A.; Huerta, F.; Trancard, J. Ischaemia of the insular cortex increases the vagal contribution to the baroreceptor reflex in the rat. J. Hypertens. 1989, 7, S36–S37. [Google Scholar] [CrossRef] [PubMed]
- Poppa, T.; Benschop, L.; Horczak, P.; Vanderhasselt, M.-A.; Carrette, E.; Bechara, A.; Baeken, C.; Vonck, K. Auricular transcutaneous vagus nerve stimulation modulates the heart-evoked potential. Brain Stimul. 2021, 15, 260–269. [Google Scholar] [CrossRef]
- Curtis, K.; Stewart, C.J.; Robinson, M.; Molfese, D.L.; Gosnell, S.N.; Kosten, T.R.; Petrosino, J.F.; Ii, R.D.L.G.; Salas, R. Insular resting state functional connectivity is associated with gut microbiota diversity. Eur. J. Neurosci. 2018, 50, 2446–2452. [Google Scholar] [CrossRef]
- Duan, X.; Hu, M.; Huang, X.; Su, C.; Zong, X.; Dong, X.; He, C.; Xiao, J.; Li, H.; Tang, J.; et al. Effect of Risperidone Monotherapy on Dynamic Functional Connectivity of Insular Subdivisions in Treatment-Naive, First-Episode Schizophrenia. Schizophr. Bull. 2019, 46, 650–660. [Google Scholar] [CrossRef]
- Liemburg, E.J.; van Es, F.; Knegtering, H.; Aleman, A. Effects of aripiprazole versus risperidone on brain activation during planning and social-emotional evaluation in schizophrenia: A single-blind randomized exploratory study. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 79, 112–119. [Google Scholar] [CrossRef]
- Yates, D. Retrieving immune responses stored in the insular cortex. Nat. Rev. Neurosci. 2021, 23, 2–3. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, F.; Wu, J.; Liu, C.; Yang, G.; Piao, R.; Geng, B.; Xu, K.; Liu, P. Altered structural covariance and functional connectivity of the insula in patients with Crohn’s disease. Quant. Imaging Med. Surg. 2022, 12, 1020–1036. [Google Scholar] [CrossRef]
- Haruki, Y.; Ogawa, K. Role of anatomical insular subdivisions in interoception: Interoceptive attention and accuracy have dissociable substrates. Eur. J. Neurosci. 2021, 53, 2669–2680. [Google Scholar] [CrossRef]
- Goudot, C.; Coillard, A.; Villani, A.-C.; Gueguen, P.; Cros, A.; Sarkizova, S.; Tang-Huau, T.-L.; Bohec, M.; Baulande, S.; Hacohen, N.; et al. Aryl Hydrocarbon Receptor Controls Monocyte Differentiation into Dendritic Cells versus Macrophages. Immunity 2017, 47, 582–596.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuber-Champier, A.; Voruz, P.; de Alcântara, I.J.; Breville, G.; Allali, G.; Lalive, P.H.; Assal, F.; Péron, J.A. Monocytosis in the Acute Phase of SARS-CoV-2 Infection Predicts the Presence of Anosognosia for Cognitive Deficits in the Chronic Phase. Brain Behav. Immun. Health 2022, 26, 100511. [Google Scholar] [CrossRef] [PubMed]
- Juengst, S.; Skidmore, E.; Pramuka, M.; McCue, M.; Becker, J. Factors contributing to impaired self-awareness of cognitive functioning in an HIV positive and at-risk population. Disabil. Rehabil. 2011, 34, 19–25. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, J.; Zhu, Y.; Yan, F.; Han, X.; Tan, Y.; Li, R. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in schizophrenia. Australas Psychiatry 2022, 30, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Melbourne, J.K.; Rosen, C.; Chase, K.A.; Feiner, B.; Sharma, R.P. Monocyte Transcriptional Profiling Highlights a Shift in Immune Signatures Over the Course of Illness in Schizophrenia. Front. Psychiatry 2021, 12, 649494. [Google Scholar] [CrossRef]
- Sahpolat, M.; Ayar, D.; Ari, M.; Karaman, M.A. Elevated Monocyte to High-density Lipoprotein Ratios as an Inflammation Markers for Schizophrenia Patients. Clin. Psychopharmacol. Neurosci. 2021, 19, 112–116. [Google Scholar] [CrossRef]
- Weber, N.S.; Gressitt, K.L.; Cowan, D.N.; Niebuhr, D.W.; Yolken, R.H.; Severance, E.G. Monocyte activation detected prior to a diagnosis of schizophrenia in the US Military New Onset Psychosis Project (MNOPP). Schizophr. Res. 2018, 197, 465–469. [Google Scholar] [CrossRef]
- Munawara, U.; Catanzaro, M.; Xu, W.; Tan, C.; Hirokawa, K.; Bosco, N.; Dumoulin, D.; Khalil, A.; Larbi, A.; Lévesque, S.; et al. Hyperactivation of monocytes and macrophages in MCI patients contributes to the progression of Alzheimer’s disease. Immun. Ageing 2021, 18, 29. [Google Scholar] [CrossRef]
- Migliorelli, R.; Tesón, A.; Sabe, L.; Petracca, G.; Petracchi, M.; Leiguarda, R.; E Starkstein, S. Anosognosia in Alzheimer’s disease: A study of associated factors. J. Neuropsychiatry 1995, 7, 338–344. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.; Shin, S.J.; Park, Y.H.; Nam, Y.; Kim, C.W.; Lee, K.W.; Kim, S.-M.; Jung, I.D.; Yang, H.D.; et al. Gram-negative bacteria and their lipopolysaccharides in Alzheimer’s disease: Pathologic roles and therapeutic implications. Transl. Neurodegener. 2021, 10, 49. [Google Scholar] [CrossRef]
- Zhao, Y.; Cong, L.; Lukiw, W.J. Lipopolysaccharide (LPS) Accumulates in Neocortical Neurons of Alzheimer’s Disease (AD) Brain and Impairs Transcription in Human Neuronal-Glial Primary Co-cultures. Front. Aging Neurosci. 2017, 9, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, A.F.; Rivest, S. Innate Immune Cells: Monocytes, Monocyte-Derived Macrophages and Microglia as Therapeutic Targets for Alzheimer’s Disease and Multiple Sclerosis. Front. Cell. Neurosci. 2019, 13, 355. [Google Scholar] [CrossRef]
- Yanuck, S.F. Microglial Phagocytosis of Neurons: Diminishing Neuronal Loss in Traumatic, Infectious, Inflammatory, and Autoimmune CNS Disorders. Front. Psychiatry 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, A.K.; Lewis, D.A.; Volk, D.W. Altered expression of microglial markers of phagocytosis in schizophrenia. Schizophr. Res. 2023, 251, 22–29. [Google Scholar] [CrossRef]
- Carson, R.E.; Naganawa, M.; Toyonaga, T.; Koohsari, S.; Yang, Y.; Chen, M.-K.; Matuskey, D.; Finnema, S.J. Imaging of Synaptic Density in Neurodegenerative Disorders. J. Nucl. Med. 2022, 63, S60–S67. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, C.; Chen, X.; Jiang, L.; Su, X. Monocytes primed with GTS-21/α7 nAChR (nicotinic acetylcholine receptor) agonist develop anti-inflammatory memory. QJM Int. J. Med. 2017, 110, 437–445. [Google Scholar] [CrossRef]
- Ghahremani, A.; Rastogi, A.; Lam, S. The Role of Right Anterior Insula and Salience Processing in Inhibitory Control. J. Neurosci. 2015, 35, 3291–3292. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, N.H.; Bechara, A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct. Funct. 2010, 214, 435–450. [Google Scholar] [CrossRef] [Green Version]
- Regner, M.F.; Tregellas, J.; Kluger, B.; Wylie, K.; Gowin, J.L.; Tanabe, J. The insula in nicotine use disorder: Functional neuroimaging and implications for neuromodulation. Neurosci. Biobehav. Rev. 2019, 103, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Walker, M.; Grace, J.; Perry, R. Acetylcholine in mind: A neurotransmitter correlate of consciousness? Trends Neurosci. 1999, 22, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Paciorek, A.; Skora, L. Vagus Nerve Stimulation as a Gateway to Interoception. Front. Psychol. 2020, 11, 1659. [Google Scholar] [CrossRef]
- Nayok, S.B.; Sreeraj, V.S.; Shivakumar, V.; Venkatasubramanian, G. A Primer on Interoception and its Importance in Psychiatry. Clin. Psychopharmacol. Neurosci. 2023, 21, 252–261. [Google Scholar] [CrossRef]
- Karczmar, A.G. Cholinergic Behaviors, Emotions, and the “Self”. J. Mol. Neurosci. 2013, 53, 291–297. [Google Scholar] [CrossRef]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Öhman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Damasio, A.; Damasio, H.; Tranel, D. Persistence of Feelings and Sentience after Bilateral Damage of the Insula. Cereb. Cortex 2012, 23, 833–846. [Google Scholar] [CrossRef] [Green Version]
- Devue, C.; Collette, F.; Balteau, E.; Degueldre, C.; Luxen, A.; Maquet, P.; Brédart, S. Here I am: The cortical correlates of visual self-recognition. Brain Res. 2007, 1143, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, N.; Saj, A.; Schwartz, S.; Ptak, R.; Schnider, A.; Thomas, C.; Conne, P.; Leroy, R.; Pavin, S.; Diserens, K.; et al. Effects of Pro-Cholinergic Treatment in Patients Suffering from Spatial Neglect. Front. Hum. Neurosci. 2013, 7, 574. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, L.; Jiang, C.; Fu, X.; Liu, X.; Wang, M.; Ou, C.; Cui, X.; Zhou, C.; Wang, J. Cerebral ischemia increases bone marrow CD4+CD25+FoxP3+ regulatory T cells in mice via signals from sympathetic nervous system. Brain Behav. Immun. 2015, 43, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Zhang, G.; Xia, Z.; Chen, N.; Yang, S.; Li, L. Identification of triazolopyridine derivatives as a new class of AhR agonists and evaluation of anti-psoriasis effect in a mouse model. Eur. J. Med. Chem. 2022, 231, 114122. [Google Scholar] [CrossRef]
- Quintana, F.J.; Sherr, D.H. Aryl Hydrocarbon Receptor Control of Adaptive Immunity. Pharmacol. Rev. 2013, 65, 1148–1161. [Google Scholar] [CrossRef] [Green Version]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.-A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Peña, G.; Cai, B.; Liu, J.; van der Zanden, E.P.; Deitch, E.A.; de Jonge, W.J.; Ulloa, L. Unphosphorylated STAT3 modulates alpha7 nicotinic receptor signaling and cytokine production in sepsis. Eur. J. Immunol. 2010, 40, 2580–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandler, N.G.; Douek, D.C. Microbial translocation in HIV infection: Causes, consequences and treatment opportunities. Nat. Rev. Microbiol. 2012, 10, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Iudicello, J.E.; Heaton, R.K.; Isnard, S.; Lin, J.; Routy, J.-P.; Gianella, S.; Hoenigl, M.; Knight, R. Markers of Gut Barrier Function and Microbial Translocation Associate with Lower Gut Microbial Diversity in People with HIV. Viruses 2021, 13, 1891. [Google Scholar] [CrossRef]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol. 2020, 11, 2148. [Google Scholar] [CrossRef]
- Kinney, D.K.; Hintz, K.; Shearer, E.M.; Barch, D.H.; Riffin, C.; Whitley, K.; Butler, R. A unifying hypothesis of schizophrenia: Abnormal immune system development may help explain roles of prenatal hazards, post-pubertal onset, stress, genes, climate, infections, and brain dysfunction. Med. Hypotheses 2010, 74, 555–563. [Google Scholar] [CrossRef]
- Khawar, M.B.; Azam, F.; Sheikh, N.; Mujeeb, K.A. How Does Interleukin-22 Mediate Liver Regeneration and Prevent Injury and Fibrosis? J. Immunol. Res. 2016, 2016, 2148129. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-J.; Gong, C.; Zhao, M.-H.; Feng, B.-S. Role of interleukin-22 in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 18177–18188. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Hsu, C.-C.; Chen, Y.-Z.; Lin, J.-J.; Tseng, H.-H.; Jang, F.-L.; Chen, P.-S.; Chen, W.-N.; Chen, C.-S.; Lin, S.-H. Profiling antibody signature of schizophrenia by Escherichia coli proteome microarrays. Brain Behav. Immun. 2022, 106, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, V. Psychosis and E. coli Infection: A Forgotten Issue. Indian J. Psychol. Med. 2012, 34, 407–408. [Google Scholar] [CrossRef] [Green Version]
- Bowie, C.R.; Reichenberg, A.; Patterson, T.L.; Heaton, R.K.; Harvey, P.D. Determinants of Real-World Functional Performance in Schizophrenia Subjects: Correlations With Cognition, Functional Capacity, and Symptoms. Am. J. Psychiatry 2006, 163, 418–425. [Google Scholar] [CrossRef]
- Kahn, R.S.; Keefe, R.S.E. Schizophrenia is a cognitive illness: Time for a change in focus. JAMA Psychiatry 2013, 70, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- McNiel, D.E.; Binder, R.L.; Robinson, J.C. Incarceration Associated With Homelessness, Mental Disorder, and Co-occurring Substance Abuse. Psychiatr. Serv. 2005, 56, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Usta, A.; Kılıç, F.; Demirdaş, A.; Işık, Ü.; Doğuç, D.K.; Bozkurt, M. Serum zonulin and claudin-5 levels in patients with schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271, 767–773. [Google Scholar] [CrossRef]
- Iwayama, Y.; Hattori, E.; Maekawa, M.; Yamada, K.; Toyota, T.; Ohnishi, T.; Iwata, Y.; Tsuchiya, K.J.; Sugihara, G.; Kikuchi, M.; et al. Association analyses between brain-expressed fatty-acid binding protein (FABP) genes and schizophrenia and bipolar disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 153B, 484–493. [Google Scholar] [CrossRef]
- Gokulakrishnan, K.; Nikhil, J.; Vs, S.; Holla, B.; Thirumoorthy, C.; Sandhya, N.; Nichenametla, S.; Pathak, H.; Shivakumar, V.; Debnath, M.; et al. Altered Intestinal Permeability Biomarkers in Schizophrenia: A Possible Link with Subclinical Inflammation. Ann. Neurosci. 2022, 29, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.T.; Lee, K.B.; Kim, S.Y.; Choi, H.R.; Park, S.C. Autophagy Impairment Induces Premature Senescence in Primary Human Fibroblasts. PLoS ONE 2011, 6, e23367. [Google Scholar] [CrossRef] [Green Version]
- Papanastasiou, E.; Gaughran, F.; Smith, S. Schizophrenia as segmental progeria. J. R. Soc. Med. 2011, 104, 475–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.-Y.; Chang, H.-W.; Lin, C.-H.; Cho, C.-L. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J. Psychiatry Neurosci. 2008, 33, 244–247. [Google Scholar]
- Negroni, A.; Cucchiara, S.; Stronati, L. Apoptosis, Necrosis, and Necroptosis in the Gut and Intestinal Homeostasis. Mediat. Inflamm. 2015, 2015, 250762. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.O. Circulating Cell-Free DNA Differentiates Severity of Inflammation. Biol. Res. Nurs. 2016, 18, 477–488. [Google Scholar] [CrossRef]
- Lubotzky, A.; Pelov, I.; Teplitz, R.; Neiman, D.; Smadja, A.; Zemmour, H.; Piyanzin, S.; Ochana, B.-L.; Spalding, K.L.; Glaser, B.; et al. Elevated brain-derived cell-free DNA among patients with first psychotic episode—A proof-of-concept study. Elife 2022, 11, e76391. [Google Scholar] [CrossRef] [PubMed]
- Zozaya-Valdés, E.; Wong, S.Q.; Raleigh, J.; Hatzimihalis, A.; Ftouni, S.; Papenfuss, A.T.; Sandhu, S.; Dawson, M.A.; Dawson, S.-J. Detection of cell-free microbial DNA using a contaminant-controlled analysis framework. Genome Biol. 2021, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zou, S.; Chu, M.; Chen, J.; Zhong, J.; Chen, Y.; Fan, J.; Qi, J.; Wang, Q. Cell free bacterial DNAs in human plasma provide fingerprints for immune-related diseases. Med. Microecol. 2020, 5, 100022. [Google Scholar] [CrossRef]
- Xiao, Q.; Lu, W.; Kong, X.; Shao, Y.W.; Hu, Y.; Wang, A.; Bao, H.; Cao, R.; Liu, K.; Wang, X.; et al. Alterations of circulating bacterial DNA in colorectal cancer and adenoma: A proof-of-concept study. Cancer Lett. 2020, 499, 201–208. [Google Scholar] [CrossRef]
- Chen, L.Y.; Qi, J.; Xu, H.L.; Lin, X.Y.; Sun, Y.J.; Ju, S.Q. The Value of Serum Cell-Free DNA Levels in Patients With Schizophrenia. Front. Psychiatry 2021, 12, 637789. [Google Scholar] [CrossRef]
- García-Bea, A.; Walker, M.A.; Hyde, T.M.; Kleinman, J.E.; Harrison, P.J.; Lane, T.A. Metabotropic glutamate receptor 3 (mGlu3; mGluR3; GRM3) in schizophrenia: Antibody characterisation and a semi-quantitative western blot study. Schizophr. Res. 2016, 177, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Arvola, M.; Keinänen, K. Characterization of the ligand-binding domains of glutamate receptor (GluR)-B and GluR-D subunits expressed in Escherichia coli as periplasmic proteins. J. Biol. Chem. 1996, 271, 15527–15532. [Google Scholar] [CrossRef] [Green Version]
- Sfera, A.; Osorio, C.; Hazan, S.; Kozlakidis, Z.; Maldonado, J.C.; del Campo, C.M.Z.-M.; Anton, J.J.; Rahman, L.; Andronescu, C.V.; Nicolson, G.L. Long COVID and the Neuroendocrinology of Microbial Translocation Outside the GI Tract: Some Treatment Strategies. Endocrines 2022, 3, 703–725. [Google Scholar] [CrossRef]
- Poland, A.; Glover, E.; Kende, A.S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976, 251, 4936–4946. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Rannug, A. The Aryl Hydrocarbon Receptor in Barrier Organ Physiology, Immunology, and Toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stejskalova, L.; Vecerova, L.; Peréz, L.M.; Vrzal, R.; Dvorak, Z.; Nachtigal, P.; Pavek, P. Aryl Hydrocarbon Receptor and Aryl Hydrocarbon Nuclear Translocator Expression in Human and Rat Placentas and Transcription Activity in Human Trophoblast Cultures. Toxicol. Sci. 2011, 123, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Wang, X.; Hawkins, B.T.; Miller, D.S. Aryl hydrocarbon receptor-mediated up-regulation of ATP-driven xenobiotic efflux transporters at the blood-brain barrier. FASEB J. 2010, 25, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Metidji, A.; Omenetti, S.; Crotta, S.; Li, Y.; Nye, E.; Ross, E.; Li, V.; Maradana, M.R.; Schiering, C.; Stockinger, B. The Environmental Sensor AHR Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity 2018, 49, 353–362.e5. [Google Scholar] [CrossRef] [Green Version]
- Tkachenko, A.; Henkler, F.; Brinkmann, J.; Sowada, J.; Genkinger, D.; Kern, C.; Tralau, T.; Luch, A. The Q-rich/PST domain of the AHR regulates both ligand-induced nuclear transport and nucleocytoplasmic shuttling. Sci. Rep. 2016, 6, 32009. [Google Scholar] [CrossRef]
- Andreeva-Gateva, P.; Bakalov, D.; Sabit, Z.; Tafradjiiska-Hadjiolova, R. Aryl hydrocarbon receptors as potential therapeutic targets. Pharmacia 2020, 67, 311–315. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Wnuk, A.; Kajta, M.; Wójtowicz, A.K. Triclosan activates aryl hydrocarbon receptor (AhR)-dependent apoptosis and affects Cyp1a1 and Cyp1b1 expression in mouse neocortical neurons. Environ. Res. 2016, 151, 106–114. [Google Scholar] [CrossRef]
- Lin, M.; Zhao, D.; Hrabovsky, A.; Pedrosa, E.; Zheng, D.; Lachman, H.M. Heat shock alters the expression of schizophrenia and autism candidate genes in an induced pluripotent stem cell model of the human telencephalon. PLoS ONE 2014, 9, e94968. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, S.J. Effects of Antipsychotic Drugs on the Heat Shock Protein 70 and 90 in the Patients with Schizophrenia. Korean Neuropsychiatr. Assoc. 2001, 40, 142–150. [Google Scholar]
- Kishinevsky, S.; Wang, T.; Rodina, A.; Chung, S.Y.; Xu, C.; Philip, J.; Taldone, T.; Joshi, S.; Alpaugh, M.L.; Bolaender, A.; et al. HSP90-incorporating chaperome networks as biosensor for disease-related pathways in patient-specific midbrain dopamine neurons. Nat. Commun. 2018, 9, 4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, Q.; Alam, M.Z.; Sait, K.H.W.; Anfinan, N.; Noorwali, A.W.; Kamal, M.A.; Khan, M.S.A.; Haque, A. Translational Shift of HSP90 as a Novel Therapeutic Target from Cancer to Neurodegenerative Disorders: An Emerging Trend in the Cure of Alzheimer’s and Parkinson’s Diseases. Curr. Drug Metab. 2017, 18, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Merchak, A.R.; Cahill, H.J.; Brown, L.C.; Brown, R.M.; Rivet-Noor, C.; Beiter, R.M.; Slogar, E.R.; Olgun, D.G.; Gaultier, A. The activity of the aryl hydrocarbon receptor in T cells tunes the gut microenvironment to sustain autoimmunity and neuroinflammation. PLoS Biol. 2023, 21, e3002000. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [Green Version]
- Herz, C.; Tran, H.T.T.; Schlotz, N.; Michels, K.; Lamy, E. Low-dose levels of bisphenol A inhibit telomerase via ER/GPR30-ERK signalling, impair DNA integrity and reduce cell proliferation in primary PBMC. Sci. Rep. 2017, 7, 16631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, P.; Prinzi, G.; Proietti, S.; Lamonaca, P.; Frustaci, A.; Boccia, S.; Amore, R.; Lorenzi, M.; Onder, G.; Marzetti, E.; et al. Shorter telomere length in schizophrenia: Evidence from a real-world population and meta-analysis of most recent literature. Schizophr. Res. 2018, 202, 37–45. [Google Scholar] [CrossRef]
- Zhang, F.; Zhen, H.; Cheng, H.; Hu, F.; Jia, Y.; Huang, B.; Jiang, M. Di-(2-ethylhexyl) phthalate exposure induces liver injury by promoting ferroptosis via downregulation of GPX4 in pregnant mice. Front. Cell Dev. Biol. 2022, 10, 1014243. [Google Scholar] [CrossRef]
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. The incidence and prevalence of schizophrenia varies with latitude. Acta Psychiatr. Scand. 2006, 114, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Eyles, D.W. Vitamin D and schizophrenia: 20 years on. Mol. Psychiatry 2021, 26, 2708–2720. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Chen, H.; Yang, J.; Fang, X.; Niu, W.; Zhang, M.; Li, J.; Pan, X.; Ren, Z.; Sun, J.; et al. Combinatory antibiotic treatment protects against experimental acute pancreatitis by suppressing gut bacterial translocation to pancreas and inhibiting NLRP3 inflammasome pathway. J. Endotoxin Res. 2019, 26, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Vasilev, A.; Sofi, R.; Tong, L.; Teschemacher, A.G.; Kasparov, S. In Search of a Breakthrough Therapy for Glioblastoma Multiforme. Neuroglia 2018, 1, 292–310. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.; Hitora, T.; Nakamura, O.; Yamagami, Y.; Horie, R.; Nishimura, H.; Yamamoto, T. Hsp90 inhibitor induces autophagy and apoptosis in osteosarcoma cells. Int. J. Oncol. 2014, 46, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boule, L.A.; Burke, C.G.; Jin, G.-B.; Lawrence, B.P. Aryl hydrocarbon receptor signaling modulates antiviral immune responses: Ligand metabolism rather than chemical source is the stronger predictor of outcome. Sci. Rep. 2018, 8, 1826. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk-Golec, K.; Pawłowska, M.; Wesołowski, R.; Wróblewski, M.; Mila-Kierzenkowska, C. Oxidative Stress as a Possible Target in the Treatment of Toxoplasmosis: Perspectives and Ambiguities. Int. J. Mol. Sci. 2021, 22, 5705. [Google Scholar] [CrossRef]
- Congdon, E.E.; Wu, J.W.; Myeku, N.; Figueroa, Y.H.; Herman, M.; Marinec, P.S.; Gestwicki, J.E.; Dickey, C.A.; Yu, W.H.; Duff, K.E. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy 2012, 8, 609–622. [Google Scholar] [CrossRef] [Green Version]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J. Exp. Clin. Cancer Res. 2021, 40, 347. [Google Scholar] [CrossRef]
- Talukdar, P.M.; Abdul, F.; Maes, M.; Binu, V.; Venkatasubramanian, G.; Kutty, B.M.; Debnath, M. Maternal Immune Activation Causes Schizophrenia-like Behaviors in the Offspring through Activation of Immune-Inflammatory, Oxidative and Apoptotic Pathways, and Lowered Antioxidant Defenses and Neuroprotection. Mol. Neurobiol. 2020, 57, 4345–4361. [Google Scholar] [CrossRef]
- Vitetta, L.; Vitetta, G.; Hall, S. Immunological Tolerance and Function: Associations Between Intestinal Bacteria, Probiotics, Prebiotics, and Phages. Front. Immunol. 2018, 9, 2240. [Google Scholar] [CrossRef] [Green Version]
- Al-Amin, M.; Uddin, M.M.N.; Reza, H.M. Effects of Antipsychotics on the Inflammatory Response System of Patients with Schizophrenia in Peripheral Blood Mononuclear Cell Cultures. Clin. Psychopharmacol. Neurosci. 2013, 11, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcinowicz, P.; Więdłocha, M.; Zborowska, N.; Dębowska, W.; Podwalski, P.; Misiak, B.; Tyburski, E.; Szulc, A. A Meta-Analysis of the Influence of Antipsychotics on Cytokines Levels in First Episode Psychosis. J. Clin. Med. 2021, 10, 2488. [Google Scholar] [CrossRef] [PubMed]
- De-Paula, V.J.; Polho, G.B.; Cardillo, G.M.; Kerr, D.S.; Chile, T.; Gattaz, W.F.; Forlenza, O.V.; Brentani, H.P. Antipsychotics preserve telomere length in peripheral blood mononuclear cells after acute oxidative stress injury. Neural Regen. Res. 2022, 17, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, E.C. Repurposing psychiatric medicines to target activated microglia in anxious mild cognitive impairment and early Parkinson’s disease. Am. J. Neurodegener. Dis. 2016, 5, 29–51. [Google Scholar] [PubMed]
- Ling, X.; Yang, W.; Zou, P.; Zhang, G.; Wang, Z.; Zhang, X.; Chen, H.; Peng, K.; Han, F.; Liu, J.; et al. TERT regulates telomere-related senescence and apoptosis through DNA damage response in male germ cells exposed to BPDE in vitro and to B[a]P in vivo. Environ. Pollut. 2018, 235, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fujikawa, M.; Oguro, A.; Itoh, K.; Vogel, C.F.A.; Ishihara, Y. Involvement of the Microglial Aryl Hydrocarbon Receptor in Neuroinflammation and Vasogenic Edema after Ischemic Stroke. Cells 2021, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Peng, V.; Sudan, R.; Antonova, A.U.; Di Luccia, B.; Ohara, T.E.; Fachi, J.L.; Grajales-Reyes, G.E.; Jaeger, N.; Trsan, T.; et al. Repression of the aryl-hydrocarbon receptor prevents oxidative stress and ferroptosis of intestinal intraepithelial lymphocytes. Immunity 2023, 56, 797–812.e4. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, R.; Peng, W.; Zhao, X.; Bai, W.; Hu, C. Quercetin alleviates ferroptosis accompanied by reducing M1 macrophage polarization during neutrophilic airway inflammation. Eur. J. Pharmacol. 2023, 938, 175407. [Google Scholar] [CrossRef]
- Mahapatra, S.; Marques, T.R. Antipsychotics, versatility in action. Proc. Natl. Acad. Sci. USA 2021, 118, e2108946118. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Livne, E.; Spanier, I.; Zuk, R.; Youdim, M.B. Iron modulates neuroleptic-induced effects related to the dopaminergic system. Isr. J. Med. Sci. 1993, 29, 587–592. [Google Scholar] [PubMed]
- Kato, T.; Monji, A.; Hashioka, S.; Kanba, S. Risperidone significantly inhibits interferon-γ-induced microglial activation in vitro. Schizophr. Res. 2007, 92, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Rácz, B.; Spengler, G. Repurposing Antidepressants and Phenothiazine Antipsychotics as Efflux Pump Inhibitors in Cancer and Infectious Diseases. Antibiotics 2023, 12, 137. [Google Scholar] [CrossRef]
- Liu, Y.; She, P.; Xu, L.; Chen, L.; Li, Y.; Liu, S.; Li, Z.; Hussain, Z.; Wu, Y. Antimicrobial, Antibiofilm, and Anti-persister Activities of Penfluridol Against Staphylococcus aureus. Front. Microbiol. 2021, 12, 727692. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Mendlovich, S.; Riwkes, S.; Braw, Y.; Levkovitch-Verbin, H.; Gal, G.; Fennig, S.; Treves, I.; Kron, S. A Double-Blind, Randomized Study of Minocycline for the Treatment of Negative and Cognitive Symptoms in Early-Phase Schizophrenia. J. Clin. Psychiatry 2009, 71, 138–149. [Google Scholar] [CrossRef] [PubMed]
- De Witte, L.D.; Laursen, T.M.; Corcoran, C.M.; Kahn, R.S.; Birnbaum, R.; Munk-Olsen, T.; Bergink, V. A Sex-Dependent Association Between Doxycycline Use and Development of Schizophrenia. Schizophr. Bull. 2023, 49, 953–961. [Google Scholar] [CrossRef]
- Fatemi, S.H. Potential microbial origins of schizophrenia and their treatments. Drugs Today 2009, 45, 305–318. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Melamud, M.M.; Buneva, V.N.; Ivanova, S.A. Immune System Abnormalities in Schizophrenia: An Integrative View and Translational Perspectives. Front. Psychiatry 2022, 13, 880568. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Dastidar, S.G.; Chakrabarty, A.N. Antimicrobial properties of methdilazine and its synergism with antibiotics and some chemotherapeutic agents. Arzneimittelforschung 1988, 38, 869–872. [Google Scholar]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafanas, E.; Schweinfurth, L.A.; Savage, C.L.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S.; et al. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav. Immun. 2017, 62, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Liu, N.; Tu, J.; Li, Z.; Han, G.; Li, J.; Sheng, C. Drug Repurposing of Haloperidol: Discovery of New Benzocyclane Derivatives as Potent Antifungal Agents against Cryptococcosis and Candidiasis. ACS Infect. Dis. 2019, 6, 768–786. [Google Scholar] [CrossRef] [PubMed]
- Villar, C.C.; Kashleva, H.; Nobile, C.J.; Mitchell, A.P.; Dongari-Bagtzoglou, A. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect. Immun. 2007, 75, 2126–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldi, S.; Codini, M.; Hunot, S.; Légeron, F.-P.; Ferri, I.; Siccu, P.; Sidoni, A.; Ambesi-Impiombato, F.S.; Beccari, T.; Curcio, F.; et al. e-Cadherin in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinson Disease. Mediat. Inflamm. 2016, 2016, 3937057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luca, A.; Zelante, T.; D’Angelo, C.; Zagarella, S.; Fallarino, F.; Spreca, A.; Iannitti, R.G.; Bonifazi, P.; Renauld, J.-C.; Bistoni, F.; et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010, 3, 361–373. [Google Scholar] [CrossRef]
- Hawi, Z.; Tong, J.; Dark, C.; Yates, H.; Johnson, B.; Bellgrove, M.A. The role of cadherin genes in five major psychiatric disorders: A literature update. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 177, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Jong, A.Y.; Stins, M.F.; Huang, S.-H.; Chen, S.H.M.; Kim, K.S. Traversal of Candida albicans across Human Blood-Brain Barrier In Vitro. Infect. Immun. 2001, 69, 4536–4544. [Google Scholar] [CrossRef] [Green Version]
- Ezeonwumelu, I.J.; Garcia-Vidal, E.; Ballana, E. JAK-STAT Pathway: A Novel Target to Tackle Viral Infections. Viruses 2021, 13, 2379. [Google Scholar] [CrossRef]
- Singh, R.K.; Dai, Y.; Staudinger, J.L.; Muma, N.A. Activation of the JAK-STAT pathway is necessary for desensitization of 5-HT2A receptor-stimulated phospholipase C signalling by olanzapine, clozapine and MDL. Int. J. Neuropsychopharmacol. 2008, 12, 651–665. [Google Scholar] [CrossRef]
- Lejeune, D.; Dumoutier, L.; Constantinescu, S.; Kruijer, W.; Schuringa, J.J.; Renauld, J.-C. Interleukin-22 (IL-22) Activates the JAK/STAT, ERK, JNK, and p38 MAP Kinase Pathways in a Rat Hepatoma Cell Line. J. Biol. Chem. 2002, 277, 33676–33682. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Kong, J.; Tan, Q.-R.; Li, X.-M. Neuroprotective effect of atypical antipsychotics in cognitive and non-cognitive behavioral impairment in animal models. Cell Adhes. Migr. 2009, 3, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Mattapallil, M.J.; Kielczewski, J.L.; Zárate-Bladés, C.; Leger, A.J.S.; Raychaudhuri, K.; Silver, P.B.; Jittayasothorn, Y.; Chan, C.-C.; Caspi, R.R. Interleukin 22 ameliorates neuropathology and protects from central nervous system autoimmunity. J. Autoimmun. 2019, 102, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Cazzullo, C.L.; Sacchetti, E.; Galluzzo, A.; Panariello, A.; Adorni, A.; Pegoraro, M.; Bosis, S.; Colombo, F.; Trabattoni, D.; Zagliani, A.; et al. Cytokine profiles in schizophrenic patients treated with risperidone: A 3-month follow-up study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Mizoguchi, Y.; Monji, A.; Horikawa, H.; Suzuki, S.O.; Seki, Y.; Iwaki, T.; Hashioka, S.; Kanba, S. Inhibitory effects of aripiprazole on interferon-induced microglial activation via intracellular Ca2+ regulation in vitro. J. Neurochem. 2008, 106, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Merenlender-Wagner, A.; Malishkevich, A.; Shemer, Z.; Udawela, M.; Gibbons, A.; Scarr, E.; Dean, B.; Levine, J.; Agam, G.; Gozes, I. Autophagy has a key role in the pathophysiology of schizophrenia. Mol. Psychiatry 2013, 20, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Mo, R.; Lai, R.; Lu, J.; Zhuang, Y.; Zhou, T.; Jiang, S.; Ren, P.; Li, Z.; Cao, Z.; Liu, Y.; et al. Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics 2018, 8, 4170–4180. [Google Scholar] [CrossRef]
- Vucicevic, L.; Misirkic-Marjanovic, M.; Harhaji-Trajkovic, L.; Maric, N.; Trajkovic, V. Mechanisms and therapeutic significance of autophagy modulation by antipsychotic drugs. Cell Stress 2018, 2, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, L. Antimicrobial IL-22. Nat. Immunol. 2017, 18, 373. [Google Scholar] [CrossRef]
- Das, S.; Croix, C.S.; Good, M.; Chen, J.; Zhao, J.; Hu, S.; Ross, M.; Myerburg, M.M.; Pilewski, J.M.; Williams, J.; et al. Interleukin-22 Inhibits Respiratory Syncytial Virus Production by Blocking Virus-Mediated Subversion of Cellular Autophagy. iScience 2020, 23, 101256. [Google Scholar] [CrossRef]
- Girgis, R.R.; Lieberman, J.A. Anti-viral properties of antipsychotic medications in the time of COVID-19. Psychiatry Res. 2020, 295, 113626. [Google Scholar] [CrossRef]
- Karwaciak, I.; Karaś, K.; Sałkowska, A.; Pastwińska, J.; Ratajewski, M. Chlorpromazine, a Clinically Approved Drug, Inhibits SARS-CoV-2 Nucleocapsid-Mediated Induction of IL-6 in Human Monocytes. Molecules 2022, 27, 3651. [Google Scholar] [CrossRef]
- Plaze, M.; Attali, D.; Petit, A.-C.; Blatzer, M.; Simon-Loriere, E.; Vinckier, F.; Cachia, A.; Chrétien, F.; Gaillard, R. Repositionnement de la chlorpromazine dans le traitement du COVID-19: Étude reCoVery. Repurposing of chlorpromazine in COVID-19 treatment: The reCoVery study. 2020, 46, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Otręba, M.; Kośmider, L.; Rzepecka-Stojko, A. Antiviral activity of chlorpromazine, fluphenazine, perphenazine, prochlorperazine, and thioridazine towards RNA-viruses. A review. Eur. J. Pharmacol. 2020, 887, 173553. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Dávola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ding, Y.; Liu, W.; Liu, S. When AHR signaling pathways meet viral infections. Cell Commun. Signal. 2023, 21, 42. [Google Scholar] [CrossRef]
- Özkucur, N.; Quinn, K.P.; Pang, J.C.; Du, C.; Georgakoudi, I.; Miller, E.; Levin, M.; Kaplan, D.L. Membrane potential depolarization causes alterations in neuron arrangement and connectivity in cocultures. Brain Behav. 2014, 5, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Skrede, S.; Holmsen, H. Har antipsykotika en rolle i membranhypotesen ved schizofreni? A role for antipsychotic agents in the membrane hypothesis of schizophrenia? Tidsskr. Nor. Laegeforen. 2003, 123, 2568–2570. [Google Scholar]
- Canfrán-Duque, A.; Barrio, L.C.; Lerma, M.; De la Peña, G.; Serna, J.; Pastor, O.; Lasunción, M.A.; Busto, R. First-Generation Antipsychotic Haloperidol Alters the Functionality of the Late Endosomal/Lysosomal Compartment in Vitro. Int. J. Mol. Sci. 2016, 17, 404. [Google Scholar] [CrossRef] [Green Version]
- Homolak, J.; Kodvanj, I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents 2020, 56, 106044. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, K.S.; Choi, Y.-K.; Lim, H.J.; Lee, H.G.; Lim, Y.; Lee, Y.H. The antipsychotic agent chlorpromazine induces autophagic cell death by inhibiting the Akt/mTOR pathway in human U-87MG glioma cells. Carcinogenesis 2013, 34, 2080–2089. [Google Scholar] [CrossRef] [Green Version]
- Katsel, P.; Davis, K.L.; Li, C.; Tan, W.; Greenstein, E.; Hoffman, L.B.K.; Haroutunian, V. Abnormal Indices of Cell Cycle Activity in Schizophrenia and their Potential Association with Oligodendrocytes. Neuropsychopharmacology 2008, 33, 2993–3009. [Google Scholar] [CrossRef] [Green Version]
- E Grant, C.; Flis, A.L.; Ryan, B.M. Understanding the role of dopamine in cancer: Past, present and future. Carcinogenesis 2022, 43, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Pan, J.; Chen, Y.; Xing, W.; Li, Q.; Wang, D.; Zhou, X.; Xie, J.; Miao, C.; Yuan, Y.; et al. Increased dopamine and its receptor dopamine receptor D1 promote tumor growth in human hepatocellular carcinoma. Cancer Commun. 2020, 40, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Boku, S.; Otsuka, I.; Mouri, K.; Aoyama, S.; Shiroiwa, K.; Sora, I.; Fujita, A.; Shirai, Y.; Shirakawa, O.; et al. The cell cycle-related genes as biomarkers for schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2016, 70, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Abrahamsen, G.; McGrath, J.J.; Mackay-Sim, A. Altered Cell Cycle Dynamics in Schizophrenia. Biol. Psychiatry 2012, 71, 129–135. [Google Scholar] [CrossRef]
- Barrio-Alonso, E.; Hernández-Vivanco, A.; Walton, C.C.; Perea, G.; Frade, J.M. Cell cycle reentry triggers hyperploidization and synaptic dysfunction followed by delayed cell death in differentiated cortical neurons. Sci. Rep. 2018, 8, 14316. [Google Scholar] [CrossRef] [Green Version]
- Mavrodi, D.V.; Blankenfeldt, W.; Thomashow, L.S. Phenazine Compounds in Fluorescent Pseudomonas Spp. Biosynthesis and Regulation. Annu. Rev. Phytopathol. 2006, 44, 417–445. [Google Scholar] [CrossRef]
- Valliappan, K.; Sun, W.; Li, Z. Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Appl. Microbiol. Biotechnol. 2014, 98, 7365–7377. [Google Scholar] [CrossRef]
- Lavaggi, M.L.; Aguirre, G.; Boiani, L.; Orelli, L.; García, B.; Cerecetto, H.; González, M. Pyrimido[1,2-a]quinoxaline 6-oxide and phenazine 5,10-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Eur. J. Med. Chem. 2008, 43, 1737–1741. [Google Scholar] [CrossRef]
- Anthérieu, S.; Azzi, P.B.-E.; Dumont, J.; Abdel-Razzak, Z.; Guguen-Guillouzo, C.; Fromenty, B.; Robin, M.-A.; Guillouzo, A. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology 2012, 57, 1518–1529. [Google Scholar] [CrossRef]
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Bock, K.W. Aryl hydrocarbon receptor (AHR) functions in infectious and sterile inflammation and NAD+-dependent metabolic adaptation. Arch. Toxicol. 2021, 95, 3449–3458. [Google Scholar] [CrossRef]
- Bernthsen, A. Ueber das Methylenblau. Berichte Dtsch Chem Ges. 1883, 16, 1025–1028. [Google Scholar] [CrossRef] [Green Version]
- Elkes, J.; Elkes, C. Effect of chlorpromazine on the behavior of chronically overactive psychotic patients. Br. Med. J. 1954, 2, 560–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, C.J.; Ávila-Gálvez, M.; Lian, Y.; Moura-Alves, P.; dos Santos, C.N. Targeting the aryl hydrocarbon receptor by gut phenolic metabolites: A strategy towards gut inflammation. Redox Biol. 2023, 61, 102622. [Google Scholar] [CrossRef] [PubMed]
- Moura-Alves, P.; Faé, K.; Houthuys, E.; Dorhoi, A.; Kreuchwig, A.; Furkert, J.; Barison, N.; Diehl, A.; Munder, A.; Constant, P.; et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512, 387–392. [Google Scholar] [CrossRef]
- Young, A.H.; Juruena, M.F.; De Zwaef, R.; Demyttenaere, K. Vagus nerve stimulation as adjunctive therapy in patients with difficult-to-treat depression (RESTORE-LIFE): Study protocol design and rationale of a real-world post-market study. BMC Psychiatry 2020, 20, 471. [Google Scholar] [CrossRef]
- Cimpianu, C.-L.; Strube, W.; Falkai, P.; Palm, U.; Hasan, A. Vagus nerve stimulation in psychiatry: A systematic review of the available evidence. J. Neural Transm. 2016, 124, 145–158. [Google Scholar] [CrossRef]
- Mogilevski, T.; Rosella, S.; Aziz, Q.; Gibson, P.R. Transcutaneous vagal nerve stimulation protects against stress-induced intestinal barrier dysfunction in healthy adults. Neurogastroenterol. Motil. 2022, 34, e14382. [Google Scholar] [CrossRef]
- Costantini, T.W.; Krzyzaniak, M.; Cheadle, G.A.; Putnam, J.G.; Hageny, A.M.; Lopez, N.; Eliceiri, B.P.; Bansal, V.; Coimbra, R. Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: A cholinergic agonist prevents gut barrier failure after severe burn injury. Am. J. Pathol. 2012, 181, 478–486. [Google Scholar] [CrossRef]
- Gautron, L.; Rutkowski, J.M.; Burton, M.D.; Wei, W.; Wan, Y.; Elmquist, J.K. Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. J. Comp. Neurol. 2013, 521, 3741–3767. [Google Scholar] [CrossRef] [Green Version]
- Goadsby, P.; Grosberg, B.; Mauskop, A.; Cady, R.; Simmons, K. Effect of noninvasive vagus nerve stimulation on acute migraine: An open-label pilot study. Cephalalgia 2014, 34, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Von Wrede, R.; Rings, T.; Bröhl, T.; Pukropski, J.; Schach, S.; Helmstaedter, C.; Lehnertz, K. Transcutaneous Auricular Vagus Nerve Stimulation Differently Modifies Functional Brain Networks of Subjects with Different Epilepsy Types. Front. Hum. Neurosci. 2022, 16, 867563. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagus Nerve Stimulation at the Interface of Brain–Gut Interactions. Cold Spring Harb. Perspect. Med. 2018, 9, a034199. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Yu, C.D.; Wang, R.; Xu, Q.J.; Dai Pra, R.; Zhang, L.; Chang, R.B. A multidimensional coding architecture of the vagal interoceptive system. Nature 2022, 603, 878–884. [Google Scholar] [CrossRef]
- Osińska, A.; Rynkiewicz, A.; Binder, M.; Komendziński, T.; Borowicz, A.; Leszczyński, A. Non-invasive Vagus Nerve Stimulation in Treatment of Disorders of Consciousness—Longitudinal Case Study. Front. Neurosci. 2022, 16, 834507. [Google Scholar] [CrossRef] [PubMed]
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2015, 8, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohleb, E.S.; Powell, N.D.; Godbout, J.P.; Sheridan, J.F. Stress-Induced Recruitment of Bone Marrow-Derived Monocytes to the Brain Promotes Anxiety-Like Behavior. J. Neurosci. 2013, 33, 13820–13833. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Latz, E.; Mills, K.H.G.; O’Neill, L.A.J. Innate immune memory: A paradigm shift in understanding host defense. Nat. Immunol. 2015, 16, 675–679. [Google Scholar] [CrossRef]
- Ahmed, U.; Graf, J.F.; Daytz, A.; Yaipen, O.; Mughrabi, I.; Jayaprakash, N.; Cotero, V.; Morton, C.; Deutschman, C.S.; Zanos, S.; et al. Ultrasound Neuromodulation of the Spleen Has Time-Dependent Anti-Inflammatory Effect in a Pneumonia Model. Front. Immunol. 2022, 13, 892086. [Google Scholar] [CrossRef]
- Bassi, G.S.; Kanashiro, A.; Coimbra, N.C.; Terrando, N.; Maixner, W.; Ulloa, L. Anatomical and clinical implications of vagal modulation of the spleen. Neurosci. Biobehav. Rev. 2020, 112, 363–373. [Google Scholar] [CrossRef]
- Dhawan, S.; De Palma, G.; Willemze, R.A.; Hilbers, F.W.; Verseijden, C.; Luyer, M.D.; Nuding, S.; Wehkamp, J.; Souwer, Y.; de Jong, E.C.; et al. Acetylcholine-producing T cells in the intestine regulate antimicrobial peptide expression and microbial diversity. Am. J. Physiol. Liver Physiol. 2016, 311, G920–G933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Kour, K.; Jayaram, M.B. Acetylcholinesterase inhibitors for schizophrenia. Cochrane Database Syst. Rev. 2012, 1, CD007967. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.; Klejbor, I.; Birkaya, B.; Lee, Y.-W.; Morys, J.; Stachowiak, E.K.; Terranova, C.; Bencherif, M.; Stachowiak, M.K. α7 Nicotinic receptor agonist reactivates neurogenesis in adult brain. Biochem. Pharmacol. 2013, 86, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.L.; Yakel, J.L. The α7 nicotinic acetylcholine receptors regulate hippocampal adult-neurogenesis in a sexually dimorphic fashion. Anat. Embryol. 2018, 224, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Coronas, V.; Arnault, P.; Jégou, J.-F.; Cousin, L.; Rabeony, H.; Clarhaut, S.; Harnois, T.; Lecron, J.-C.; Morel, F. IL-22 Promotes Neural Stem Cell Self-Renewal in the Adult Brain. Stem Cells 2023, 41, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, W.; Patro, C.P.K.; Zhao, J.; Mu, T.; Ma, Z.; Xu, J.; Ban, K.; Yi, C.; Zhou, Y. STAT3 Regulates Mouse Neural Progenitor Proliferation and Differentiation by Promoting Mitochondrial Metabolism. Front. Cell Dev. Biol. 2020, 8, 362. [Google Scholar] [CrossRef]
- Yun, S.; Reynolds, R.P.; Masiulis, I.; Eisch, A.J. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat. Med. 2016, 22, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, R.; Weinberger, D.R. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat. Rev. Neurosci. 2017, 18, 727–740. [Google Scholar] [CrossRef]
- Sheu, J.-R.; Hsieh, C.-Y.; Jayakumar, T.; Tseng, M.-F.; Lee, H.-N.; Huang, S.-W.; Manubolu, M.; Yang, C.-H. A Critical Period for the Development of Schizophrenia-Like Pathology by Aberrant Postnatal Neurogenesis. Front. Neurosci. 2019, 13, 635. [Google Scholar] [CrossRef]
- Ciofani, M.; Madar, A.; Galan, C.; Sellars, M.; Mace, K.; Pauli, F.; Agarwal, A.; Huang, W.; Parkurst, C.N.; Muratet, M.; et al. A Validated Regulatory Network for Th17 Cell Specification. Cell 2012, 151, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Ventevogel, M.S.; Sempowski, G.D. Thymic rejuvenation and aging. Curr. Opin. Immunol. 2013, 25, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Sfera, A.; Jafri, N.; Rahman, L. F-652 (Recombinant Human Interleukin-22) for Schizophrenia. Arch. Phar. Pharmacol. Res. 2023, 3, 1–6. [Google Scholar]
- Jones, B.C.; Logsdon, N.J.; Walter, M.R. Structure of IL-22 Bound to Its High-Affinity IL-22R1 Chain. Structure 2008, 16, 1333–1344. [Google Scholar] [CrossRef] [Green Version]
- Fu, G.; Zhang, W.; Dai, J.; Liu, J.; Li, F.; Wu, D.; Xiao, Y.; Shah, C.; Sweeney, J.A.; Wu, M.; et al. Increased Peripheral Interleukin 10 Relate to White Matter Integrity in Schizophrenia. Front. Neurosci. 2019, 13, 52. [Google Scholar] [CrossRef]
- Zakowicz, P.; Pawlak, J.; Kapelski, P.; Wiłkość-Dębczyńska, M.; Szałkowska, A.; Twarowska-Hauser, J.; Rybakowski, J.; Skibińska, M. Genetic association study reveals impact of interleukin 10 polymorphisms on cognitive functions in schizophrenia. Behav. Brain Res. 2021, 419, 113706. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Kim, Y.-G.; Hara, H.; Kamada, N.; Caballero-Flores, G.; Tolosano, E.; Soares, M.P.; Puente, J.L.; Inohara, N.; Núñez, G. IL-22 controls iron-dependent nutritional immunity against systemic bacterial infections. Sci. Immunol. 2017, 2, eaai8371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.F.; Subramaniam, V.N. Analysis of IL-22 contribution to hepcidin induction and hypoferremia during the response to LPS in vivo. Int. Immunol. 2015, 27, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Chen, J.; Qu, C.; Yang, L.; Wu, X.; Wang, S.; Yang, T.; Liu, H.; Fang, Y.; Sun, P. Identification of Ferroptosis-Related Genes in Schizophrenia Based on Bioinformatic Analysis. Genes 2022, 13, 2168. [Google Scholar] [CrossRef]
- Lotan, A.; Luza, S.; Opazo, C.M.; Ayton, S.; Lane, D.J.R.; Mancuso, S.; Pereira, A.; Sundram, S.; Weickert, C.S.; Bousman, C.; et al. Perturbed iron biology in the prefrontal cortex of people with schizophrenia. Mol. Psychiatry 2023, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Huang, H.; Zhou, S.-M.; Tian, H.-J.; Li, P. Excessive Iron Availability Caused by Disorders of Interleukin-10 and Interleukin-22 Contributes to High Altitude Polycythemia. Front. Physiol. 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Arvedson, T.L.; Cooke, K.S.; Dickmann, L.J.; Forte, C.; Li, H.; Merriam, K.L.; Perry, V.K.; Tran, L.; Rottman, J.B.; et al. IL-22 Regulates Iron Availability In Vivo through the Induction of Hepcidin. J. Immunol. 2013, 191, 1845–1855. [Google Scholar] [CrossRef] [Green Version]
- Kugelberg, E. IL-22 controls iron scavenging. Nat. Rev. Immunol. 2017, 17, 146–147. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, C.; North, H.F.; Bitar, M.; Fullerton, J.M.; Sager, R.; Barry, G.; Piper, M.; Halliday, G.M.; Webster, M.J.; Weickert, C.S. Reduced adult neurogenesis is associated with increased macrophages in the subependymal zone in schizophrenia. Mol. Psychiatry 2021, 26, 6880–6895. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-Y.; Lee, H.; Dingle, R.W.C.; Kim, K.B.; Swanson, H.I. Development of Novel CH223191-Based Antagonists of the Aryl Hydrocarbon Receptor. Mol. Pharmacol. 2011, 81, 3–11. [Google Scholar] [CrossRef] [Green Version]
- McGovern, K.; Castro, A.C.; Cavanaugh, J.; Coma, S.; Walsh, M.; Tchaicha, J.; Syed, S.; Natarajan, P.; Manfredi, M.; Zhang, X.M.; et al. Discovery and Characterization of a Novel Aryl Hydrocarbon Receptor Inhibitor, IK-175, and Its Inhibitory Activity on Tumor Immune Suppression. Mol. Cancer Ther. 2022, 21, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Han, H.; Goldsby, J.; Mohankumar, K.; Chapkin, R.S. Aryl hydrocarbon receptor (AhR) ligands as selective AhR modulators: Genomic studies. Curr. Opin. Toxicol. 2018, 11–12, 10–20. [Google Scholar] [CrossRef]
- Bartoňková, I.; Dvořák, Z. Essential oils of culinary herbs and spices display agonist and antagonist activities at human aryl hydrocarbon receptor AhR. Food Chem. Toxicol. 2018, 111, 374–384. [Google Scholar] [CrossRef]
- Goya-Jorge, E.; Rodríguez, M.E.J.; Veitía, M.S.-I.; Giner, R.M. Plant Occurring Flavonoids as Modulators of the Aryl Hydrocarbon Receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef]
- Zatloukalová, J.; Švihálková-Šindlerová, L.; Kozubík, A.; Krčmář, P.; Machala, M.; Vondráček, J. β-Naphthoflavone and 3′-methoxy-4′-nitroflavone exert ambiguous effects on Ah receptor-dependent cell proliferation and gene expression in rat liver ‘stem-like’ cells. Biochem. Pharmacol. 2007, 73, 1622–1634. [Google Scholar] [CrossRef]
- Wang, Q.; VonHandorf, A.; Puga, A. Aryl Hydrocarbon Receptor. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Tian, J.; Feng, Y.; Fu, H.; Xie, H.Q.; Jiang, J.X.; Zhao, B. The Aryl Hydrocarbon Receptor: A Key Bridging Molecule of External and Internal Chemical Signals. Environ. Sci. Technol. 2015, 49, 9518–9531. [Google Scholar] [CrossRef] [Green Version]
- DeRosa, H.; Richter, T.; Wilkinson, C.; Hunter, R.G. Bridging the Gap Between Environmental Adversity and Neuropsychiatric Disorders: The Role of Transposable Elements. Front. Genet. 2022, 13, 813510. [Google Scholar] [CrossRef]
- Munro, A.W. Cytochrome P450 1A1 opens up to new substrates. J. Biol. Chem. 2018, 293, 19211–19212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.-X.; Tang, X.; Zhu, J.-Y.; Luo, L.; Ma, X.-Y.; Cheng, S.-W.; Zhang, W.; Tang, W.-Q.; Ma, W.; Yang, X.; et al. Cytochrome P450 1A1 enhances inflammatory responses and impedes phagocytosis of bacteria in macrophages during sepsis. Cell Commun. Signal. 2020, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Fizíková, I.; Dragašek, J.; Račay, P. Mitochondrial Dysfunction, Altered Mitochondrial Oxygen, and Energy Metabolism Associated with the Pathogenesis of Schizophrenia. Int. J. Mol. Sci. 2023, 24, 7991. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Interactions of Tryptophan and Its Catabolites With Melatonin and the Alpha 7 Nicotinic Receptor in Central Nervous System and Psychiatric Disorders: Role of the Aryl Hydrocarbon Receptor and Direct Mitochondria Regulation. Int. J. Tryptophan Res. 2017, 10, 1178646917691738. [Google Scholar] [CrossRef]
- Hare, S.M.; Adhikari, B.M.; Mo, C.; Chen, S.; Wijtenburg, S.A.; Seneviratne, C.; Kane-Gerard, S.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Schwarcz, R.; et al. Tryptophan challenge in individuals with schizophrenia and healthy controls: Acute effects on circulating kynurenine and kynurenic acid, cognition and cerebral blood flow. Neuropsychopharmacology 2023, 1–8. [Google Scholar] [CrossRef]
- Mokkawes, T.; de Visser, S.P. Melatonin Activation by Cytochrome P450 Isozymes: How Does CYP1A2 Compare to CYP1A1? Int. J. Mol. Sci. 2023, 24, 3651. [Google Scholar] [CrossRef]
- Jang, S.W.; Liu, X.; Pradoldej, S.; Tosini, G.; Chang, Q.; Iuvone, P.M.; Ye, K. N-acetylserotonin activates TrkB receptor in a cir-cadian rhythm. Proc. Natl. Acad. Sci. USA 2010, 107, 3876. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Gut Dysbiosis Dysregulates Central and Systemic Homeostasis via Suboptimal Mitochondrial Function: Assessment, Treatment and Classification Implications. Curr. Top. Med. Chem. 2020, 20, 524–539. [Google Scholar] [CrossRef] [PubMed]



| DH-Discordant SCZ Features | Non-DA Mechanisms | References |
|---|---|---|
| Negative symptoms | Translocation of Hafnei alvei, Pseudomonas aeruginosa, Morganella morganii, Pseudomonas putida, and Klebsiella pneumoniae | [12,13,14] |
| Comorbidity with IBD | AhR/STAT3/IL-22-regulated intestinal permeability and microbiota translocation | [11,15,16] |
| Comorbidity with HIV | AhR/STAT3/IL22-regulateted gut barrier permeability | [12,17,18] |
| Poor insight (anosognosia) | IC activation by gut Prevotella and Bacteroides abundance | [19,20,21,22,23,24,25] |
| Higher prevalence in urban areas | Pollutants are AhR ligands associated with SCZ and are more prevalent in industrialized countries and urban areas | [26,27,28,29,30,31,32,33,34] |
| Increasing prevalence with the distance from the equator | Sunlight-driven vitamin D derivatives and tryptophan light metabolites are AhR ligands | [35,36,37,38,39] |
| Autoantibodies | Gut microbes express molecules, including GABA and NMDA, which can elicit formation of antibodies upon translocation | [40,41,42] |
| Exogenous Agonists | Endogenous Agonists | Dietary Antagonists | Pharmacological Antagonists |
|---|---|---|---|
| Benzotriazole UV stabilizer | Tryptophan photo metabolites and FICZ | quercetin | CH-223191 |
| Plasticizers (Bisphenol) | Indoles | apigenin | Alpha-naphtoflavone 3-methroxy-4-itriflavone |
| Clozapine | D3 hydroxyderivatives | luteolin | BAY2416964 |
| Carbidopa | DA | kaempferol | HBU651 |
| Antipsychotic Drugs | Recombinant IL-22 | References |
|---|---|---|
| JAK-STAT activation | JAK-STAT activation | [239,240] |
| Neuroprotective | Neuroprotective | [241,242] |
| IFN-γ inhibitor | IFN-γ inhibitor | [243,244] |
| Activate autophagy | Activate autophagy | [245,246,247] |
| Antibacterial/antiviral | Antibacterial/antiviral | [230,248,249,250] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfera, A. Six Decades of Dopamine Hypothesis: Is Aryl Hydrocarbon Receptor the New D2? Reports 2023, 6, 36. https://doi.org/10.3390/reports6030036
Sfera A. Six Decades of Dopamine Hypothesis: Is Aryl Hydrocarbon Receptor the New D2? Reports. 2023; 6(3):36. https://doi.org/10.3390/reports6030036
Chicago/Turabian StyleSfera, Adonis. 2023. "Six Decades of Dopamine Hypothesis: Is Aryl Hydrocarbon Receptor the New D2?" Reports 6, no. 3: 36. https://doi.org/10.3390/reports6030036
APA StyleSfera, A. (2023). Six Decades of Dopamine Hypothesis: Is Aryl Hydrocarbon Receptor the New D2? Reports, 6(3), 36. https://doi.org/10.3390/reports6030036