Phosphate Sorption Speciation and Precipitation Mechanisms on Amorphous Aluminum Hydroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Amorphous Al(OH)3 and Amorphous AlPO4
2.2. Phosphate Sorption Isotherms and Kinetics
2.3. XRD, PDF and Infrared Spectroscopy
2.4. Phosphorus K-Edge XANES Spectroscopy
2.5. 27Al and 31P NMR Spectroscopy
3. Results
3.1. Sorption Kinetics and Isotherms
3.2. X-ray Diffraction
3.3. Pair Distribution Function Analyses
3.4. Phosphorus K-Edge XANES Spectroscopy
3.5. 31P and 27Al NMR Spectra
3.6. ATR-FTIR Spectroscopy
4. Discussion
4.1. Structure of Phosphate Surface Complexes on AAH
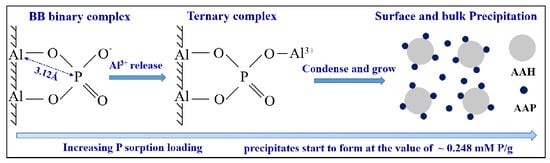
4.2. Quantitative Relationship between P Sorbed and P Precipitated
4.3. AlIII Release during Phosphate Sorption and Formation Mechanisms of AAP
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, Y.; Lu, J. Effect of phosphorus fertilization on yield and phosphorus use efficiency of winter oilseed rape (Brassica napus L.) with two different cropping intensities in the middle and lower reaches of Yangtze River. J. Food Agric. Environ. 2012, 10, 576–579. [Google Scholar]
- Bromfield, S.M. Relative contribution of iron and aluminium in phosphate sorption by acid surface soils. Nature 1964, 201, 321–322. [Google Scholar] [CrossRef]
- Bortoluzzi, E.C.; Perez, C.A.S.; Ardisson, J.D.; Tiecher, T.; Caner, L. Occurrence of iron and aluminum sesquioxides and their implications for the P sorption in subtropical soils. Appl. Clay Sci. 2015, 104, 196–204. [Google Scholar] [CrossRef]
- Freese, D.; Vanderzee, S.E.A.T.M.; Vanriemsdijk, W.H. Comparison of different models for phosphate sorption as a function of the iron and aluminum-oxides of soils. J. Soil Sci. 1992, 43, 729–738. [Google Scholar] [CrossRef]
- Gerard, F. Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils—A myth revisited. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Liang, X.; Zhao, Y.; Cade-Menun, B.J.; Hu, Y. Molecular speciation of phosphorus present in readily dispersible colloids from agricultural soils. Soil Sci. Soc. Am. J. 2014, 78, 47–53. [Google Scholar] [CrossRef]
- Kumar, E.; Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpaa, M. Interaction of inorganic anions with iron-mineral adsorbents in aqueous media—A review. Adv. Colloid Interface 2014, 203, 11–21. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Tan, W.; Li, W.; Feng, X.; Sparks, D.L. Characteristics of phosphate adsorption-desorption onto ferrihydrite: Comparison with well-crystalline Fe (hydr)oxides. Soil Sci. 2013, 178, 1–11. [Google Scholar] [CrossRef]
- Mallet, M.; Barthelemy, K.; Ruby, C.; Renard, A.; Naille, S. Investigation of phosphate adsorption onto ferrihydrite by X-ray photoelectron spectroscopy. J. Colloid Interface Sci. 2013, 407, 95–101. [Google Scholar] [CrossRef]
- Kim, J.; Li, W.; Philips, B.L.; Grey, C.P. Phosphate adsorption on the iron oxyhydroxides goethite (α-FeOOH), akaganeite (β-FeOOH), and lepidocrocite (γ-FeOOH): A 31P NMR study. Energy Environ. Sci. 2011, 4, 4298–4305. [Google Scholar] [CrossRef]
- Celi, L.; De Luca, G.; Barberis, E. Effects of interaction of organic and inorganic P with ferrihydrite and kaolinite-iron oxide systems on iron release. Soil Sci. 2003, 168, 479–488. [Google Scholar] [CrossRef]
- Rajan, S.S.S. Adsorption of divalent phosphate on hydrous aluminum-oxide. Nature 1975, 253, 434–436. [Google Scholar] [CrossRef]
- Shang, C.; Stewart, J.W.B.; Huang, P. PH effect on kinetics of adsorption of organic and inorganic phosphates by short-range ordered aluminum and iron precipitates. Geoderma 1992, 53, 1–14. [Google Scholar] [CrossRef]
- Jain, A.; Raven, K.P.; Loeppert, R.H. Arsenite and arsenate adsorption on ferrihydrite: Surface charge reduction and net OH− release stoichiometry. Environ. Sci. Technol. 1999, 33, 1179–1184. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Harrington, R.; Liu, F.; Parise, J.B.; Feng, X.; Sparks, D.L. Effect of ferrihydrite crystallite size on phosphate adsorption reactivity. Environ. Sci. Technol. 2013, 47, 10322–10331. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Tang, Y.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces. Environ. Sci. Nano 2017, 4, 2193–2204. [Google Scholar] [CrossRef]
- Li, W.; Pierre-Louis, A.M.; Kwon, K.D.; Kubicki, J.D.; Strongin, D.R.; Phillips, B.L. Molecular level investigations of phosphate sorption on corundum (α-Al2O3) by 31P solid state NMR, ATR-FTIR and quantum chemical calculation. Geochim. Cosmochim. Acta 2013, 107, 252–266. [Google Scholar] [CrossRef]
- Li, W.; Feng, X.; Yan, Y.; Sparks, D.L.; Phillips, B.L. Solid-state NMR spectroscopic study of phosphate sorption mechanisms on aluminum (hydr)oxides. Environ. Sci. Technol. 2013, 47, 8308–8315. [Google Scholar] [CrossRef]
- Li, W.; Feng, J.; Kwon, K.D.; Kubicki, J.D.; Phillips, B.L. Surface Speciation of Phosphate on Boehmite (gamma-AlOOH) Determined from NMR Spectroscopy. Langmuir 2010, 26, 4753–4761. [Google Scholar] [CrossRef]
- Wang, L.; Putnis, C.V.; Ruiz-Agudo, E.; Hovelmann, J.; Putnis, A. In situ imaging of interfacial precipitation of phosphate on goethite. Environ. Sci. Technol. 2015, 49, 4184–4192. [Google Scholar] [CrossRef]
- Yan, Y.; Li, W.; Yang, J.; Zheng, A.; Liu, F.; Feng, X.; Sparks, D.L. Mechanism of myo-inositol hexakisphosphate sorption on amorphous aluminum hydroxide: Spectroscopic evidence for rapid surface precipitation. Environ. Sci. Technol. 2014, 48, 6735–6742. [Google Scholar] [CrossRef] [PubMed]
- Lookman, R.; Grobet, P.; Merckx, R.; Vlassak, K. Phosphate sorption by synthetic amorphous aluminum hydroxides: A 27Al and 31P solid-state MAS NMR spectroscopy study. Eur. J. Soil Sci. 1994, 45, 37–44. [Google Scholar] [CrossRef]
- Kim, Y.; Kirkpatrick, R.J. An investigation of phosphate adsorbed on aluminium oxyhydroxide and oxide phases by nuclear magnetic resonance. Eur. J. Soil Sci. 2004, 55, 243–251. [Google Scholar] [CrossRef]
- Van Emmerik, T.J.; Sandstrom, D.E.; Antzutkin, O.N.; Angove, M.J.; Johnson, B.B. 31P solid-state nuclear magnetic resonance study of the sorption of phosphate onto gibbsite and kaolinite. Langmuir 2007, 23, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Lookman, R.; Grobet, P.; Merckx, R.; Van Riemsdijk, W.H. Application of P-31 and Al-27 MAS NMR for phosphate speciation studies in soil and aluminium hydroxides: Promises and constraints. Geoderma 1997, 80, 369–388. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, F.; Li, W.; Liu, F.; Feng, X.; Sparks, D.L. Sorption and desorption characteristics of organic phosphates of different structures on aluminium (oxyhydr)oxides. Eur. J. Soil Sci. 2014, 65, 308–317. [Google Scholar] [CrossRef]
- Yan, X.; Wei, Z.Q.; Hong, Q.Q.; Lu, Z.H.; Wu, J.F. Phosphorus fractions and sorption characteristics in a subtropical paddy soil as influenced by fertilizer sources. Geoderma 2017, 295, 80–85. [Google Scholar] [CrossRef]
- Pierzynski, J.; Hettiarachchi, G.M. Reactions of phosphorus fertilizers with and without a fertilizer enhancer in three acidic soils with high phosphorus-fixing capacity. Soil Sci. Soc. Am. J. 2018, 82, 1124–1139. [Google Scholar] [CrossRef]
- Wang, X.; Gu, C.; Feng, X.; Zhu, M. Sulfate local coordination environment in schwertmannite. Environ. Sci. Technol. 2015, 49, 10440–10448. [Google Scholar] [CrossRef] [PubMed]
- Jaumot, J.; Gargallo, R.; de Juan, A.; Tauler, R. A graphical user-friendly interface for MCR-ALS: A new tool for multivariate curve resolution in MATLAB. Chemometr. Intell. Lab. 2005, 76, 101–110. [Google Scholar] [CrossRef]
- Wang, X.; Kubicki, J.D.; Boily, J.F.; Waychunas, G.A.; Hu, Y.; Feng, X.; Zhu, M. Binding geometries of silicate species on ferrihydrite surfaces. ACS Earth Space Chem. 2018, 2, 125–134. [Google Scholar] [CrossRef]
- Webb, S.M. SIXpack: A graphical user interface for XAS analysis using IFEFFIT. Phys. Scr. 2005, T115, 1011–1014. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, D.; Zhou, W.Q.; Hutchison, K.J.; Beauchemin, S.; Sayers, D.E. XAFS study of adsorbed and mineral forms of phosphate. J. Synchrotron Radiat. 1999, 6, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Sparks, D.L. Phosphate reaction dynamics in soils and soil components: A multiscale approach. Adv. Agron. 2007, 94, 135–179. [Google Scholar]
- Khare, N.; Martin, J.D.; Hesterberg, D. Phosphate bonding configuration on ferrihydrite based on molecular orbital calculations and XANES fingerprinting. Geochim. Cosmochim. Acta 2007, 71, 4405–4415. [Google Scholar] [CrossRef]
- Mortlock, R.F.; Bell, A.T.; Radke, C.J. 31P and 27Al NMR investigations of the effects of pH on aqueous solutions containing aluminum and phosphorus. J. Phys. Chem. 1993, 97, 775–782. [Google Scholar] [CrossRef]
- Isobe, T.; Watanabe, T.; de la Caillerie, J.B.D.; Legrand, A.P.; Massiot, D. Solid-state H-1 and Al-27 NMR studies of amorphous aluminum hydroxides. J. Colloid Interface Sci. 2003, 261, 320–324. [Google Scholar] [CrossRef]
- Li, W.; Harrington, R.; Tang, Y.; Kubicki, J.D.; Aryanpour, M.; Reeder, R.J.; Parise, J.B.; Phillips, B.L. Differential pair distribution function study of the structure of arsenate adsorbed on nanocrystalline γ-Alumina. Environ. Sci. Technol. 2011, 45, 9687–9692. [Google Scholar] [CrossRef]
- van Genuchten, C.M.; Pena, J. Sorption selectivity of birnessite particle edges: A d-PDF analysis of Cd(II) and Pb(II) sorption by δ-MnO2 and ferrihydrite. Environ. Sci. Proc. Impacts 2016, 18, 1030–1041. [Google Scholar] [CrossRef]
- Zhu, M.; Northrup, P.; Shi, C.; Billinge, S.J.L.; Sparks, D.L.; Waychunas, G.A. Structure of sulfate adsorption complexes on ferrihydrite. Environ. Sci. Technol. Lett. 2014, 1, 97–101. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Koopal, L.K.; Li, W.; Xu, W.; Liu, F.; Zhang, J.; Liu, Q.; Feng, X.; Sparks, D.L. Effects of crystallite size on the structure and magnetism of ferrihydrite. Environ. Sci. Nano 2016, 3, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Khare, N.; Hesterberg, D.; Martin, J.D. XANES investigation of phosphate sorption in single and binary systems of iron and aluminum oxide minerals. Environ. Sci. Technol. 2005, 39, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hesterberg, D. Phosphate bonding on noncrystalline Al/Fe-hydroxide coprecipitates. Environ. Sci. Technol. 2011, 45, 6283–6289. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGrawHill: New York, NY, USA, 1999. [Google Scholar]
- Xie, R.; Gomez, M.J.; Xing, Y.; Klose, P.S. Fouling assessment in a municipal water reclamation reverse osmosis system as related to concentration factor. J. Environ. Eng. Sci. 2004, 3, 61–72. [Google Scholar] [CrossRef]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Kennedy, D.W. Biotransformation of two-line silica-ferrihydrite by a dissimilatory Fe(III)-reducing bacterium: Formation of carbonate green rust in the presence of phosphate. Geochim. Cosmochim. Acta 2004, 68, 2799–2814. [Google Scholar] [CrossRef]
- Stumm, W. Reactivity at the mineral-water interface: Dissolution and inhibition. Colloids Surf. A Physicochem. Eng. Asp. 1997, 120, 143–166. [Google Scholar] [CrossRef]
- Del Nero, M.; Galindo, C.; Barillon, R.; Halter, E.; Made, B. Surface reactivity of α-Al2O3 and mechanisms of phosphate sorption: In situ ATR-FTIR spectroscopy and zeta potential studies. J. Colloid Interface Sci. 2010, 342, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Bomans, P.H.H.; Muller, F.A.; Will, J.; Frederik, P.M.; de With, G.; Sommerdijk, N.A.J.M. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater. 2010, 9, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Putnis, A. Why mineral interfaces matter. Science 2014, 343, 1441–1442. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Agudo, E.; Putnis, C.V.; Putnis, A. Coupled dissolution and precipitation at mineral-fluid interfaces. Chem. Geol. 2014, 383, 132–146. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V.; Rodriguez-Navarro, C.; Putnis, A. Mechanism of leached layer formation during chemical weathering of silicate minerals. Geology 2012, 40, 947–950. [Google Scholar] [CrossRef]
- Nooney, M.G.; Campbell, A.; Murrell, T.S.; Lin, X.; Hossner, L.R.; Chusuei, C.C.; Goodman, D.W. Nucleation and growth of phosphate on metal oxide thin films. Langmuir 1998, 14, 2750–2755. [Google Scholar] [CrossRef]
- Huang, L.; Moore, P.A.; Kleinman, P.J.A.; Elkin, K.R.; Savin, M.C.; Pote, D.H.; Edwards, D.R. Reducing phosphorus runoff and leaching from poultry litter with alum: Twenty-year small plot and paired-watershed studies. J. Environ. Qual. 2016, 45, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Antelo, J.; Fiol, S.; Perez, C.; Marino, S.; Arce, F.; Gondar, D.; Lopez, R. Analysis of phosphate adsorption onto ferrihydrite using the CD-MUSIC model. J. Colloid Interface Sci. 2010, 347, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.V.; Rhoton, F.E.; Selim, H.M. Modeling the impact of ferrihydrite on adsorption-desorption of soil phosphorus. Soil Sci. 2004, 169, 271–281. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Phillips, B.L.; Boily, J.-F.; Hu, Y.; Hu, Z.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate Sorption Speciation and Precipitation Mechanisms on Amorphous Aluminum Hydroxide. Soil Syst. 2019, 3, 20. https://doi.org/10.3390/soilsystems3010020
Wang X, Phillips BL, Boily J-F, Hu Y, Hu Z, Yang P, Feng X, Xu W, Zhu M. Phosphate Sorption Speciation and Precipitation Mechanisms on Amorphous Aluminum Hydroxide. Soil Systems. 2019; 3(1):20. https://doi.org/10.3390/soilsystems3010020
Chicago/Turabian StyleWang, Xiaoming, Brian L. Phillips, Jean-François Boily, Yongfeng Hu, Zhen Hu, Peng Yang, Xionghan Feng, Wenqian Xu, and Mengqiang Zhu. 2019. "Phosphate Sorption Speciation and Precipitation Mechanisms on Amorphous Aluminum Hydroxide" Soil Systems 3, no. 1: 20. https://doi.org/10.3390/soilsystems3010020
APA StyleWang, X., Phillips, B. L., Boily, J. -F., Hu, Y., Hu, Z., Yang, P., Feng, X., Xu, W., & Zhu, M. (2019). Phosphate Sorption Speciation and Precipitation Mechanisms on Amorphous Aluminum Hydroxide. Soil Systems, 3(1), 20. https://doi.org/10.3390/soilsystems3010020