Effects of Agricultural Expansion on Soil Carbon and Nitrogen Stocks in the Amazon Deforestation Arc
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Study Site
2.2. Experimental Characterization of the System
2.3. Chronosequences Validation
2.4. Soil Sampling
2.5. Concentration and Stock of C and N
2.6. Soil Stocks Calculations
2.7. Light Organic Matter (LOM) in Water
2.8. Statistical Analysis
3. Results
3.1. Chronosequence Validation
3.2. Organic C and Soil N
3.3. C Origin
3.4. Light Organic Matter and C:N Ratio
4. Discussion
4.1. Chronosequence Validation
4.2. Organic C and Soil N
4.3. C Origin
4.4. Light Organic Matter and C:N Ratio
4.5. Challenges and Gaps in Adopting the Chronosequence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berenguer, E.; Ferreira, J.; Gardner, T.A.; Aragão, L.E.O.C.; Camargo, P.B.; Cerri, C.E.; Durigan, M.; Oliveira Junior, R.C.; Vieira, I.C.G.; Barlow, J. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Chang. Biol. 2014, 20, 3713–3726. [Google Scholar] [CrossRef]
- Fujisaki, K.; Perrin, A.S.; Desjardins, T.; Bernoux, M.; Balbino, L.C.; Brossard, M. From forest to cropland and pasture systems: A critical review of soil organic carbon stocks changes in Amazonia. Glob. Chang. Biol. 2015, 21, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Sonter, L.J.; Herrera, D.; Barrett, D.J.; Galford, G.L.; Moran, C.J.; Soares Filho, B.S. Mining drives extensive deforestation in the Brazilian Amazon. Nat. Com. 2017, 8, 1013. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.F.; Fulginiti, L.E.; Perrin, R.K. The Cost of Forest Preservation in the Brazilian Amazon: The “Arc of Deforestation”. J. Agri. Resour. Econ. 2019, 44, 497–512. [Google Scholar] [CrossRef]
- Hargrave, J.; Kis-Kato, K. Economic Causes of Deforestation in the Brazilian Amazon: A Panel Data Analysis for the 2000s. Environ. Resour. Econ. 2013, 54, 471–494. [Google Scholar] [CrossRef]
- Santos, C.A.; Rezende, C.P.; Pinheiro, E.F.; Pereira, J.M.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Changes in soil carbon stocks after land-use change from native vegetation to pastures in the Atlantic forest region of Brazil. Geoderma 2019, 337, 394–401. [Google Scholar] [CrossRef]
- Gatti, L.V.; Basso, L.S.; Miller, J.B.; Gloor, M.; Domingues, L.G.; Cassol, H.L.G.; Tejeda, G.; Aragão, L.E.O.C.; Nobre, C.; Peters, W.; et al. Amazonia is a carbon source linked to deforestation and climate change. Nature 2021, 595, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Salimon, C.I.; Wadt, P.G.S.; Alves, S.S. Decrease in carbon stocks in an oxisol due to land use and cover change in southwestern Amazon. Ambi-Agua 2009, 4, 57–65. [Google Scholar] [CrossRef]
- Braz, S.P.; Urquiaga, S.; Alves, B.J.R.; Jantalia, C.P.; Guimarães, A.P.; dos Santos, C.A.; dos Santos, S.C.; Pinheiro, E.M.; Boddey, R.M. Soil carbon stocks under productive and degraded Brachiaria pastures in the Brazilian Cerrados. Soil Sci. Soc. Am. J. 2013, 77, 914–928. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks–a meta-analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, G.; Tang, Z.; Shangguan, Z. Global patterns of the effects of land-use changes on soil carbon stocks. Glob. Ecol. Conser. 2016, 5, 127–138. [Google Scholar] [CrossRef]
- Frazão, L.A.; Paustian, K.; Cerri, C.E.P.; Cerri, C.C. Soil carbon stocks and changes after oil palm introduction in the Brazilian Amazon. Glob. Chang. Biol. Bioenergy 2013, 5, 384–390. [Google Scholar] [CrossRef]
- Rittl, T.F.; Oliveira, D.; Cerri, C.E.P. Soil carbon stock changes under different land uses in the Amazon. Geoderma Reg. 2017, 10, 138–143. [Google Scholar] [CrossRef]
- Navarrete, D.; Sitch, S.; Aragão, L.E.O.C.; Pedroni, L. Conversion from forests to pastures in the Colombian Amazon leads to contrasting soil carbon dynamics depending on land management practices. Glob. Chang. Biol. 2016, 22, 3503–3517. [Google Scholar] [CrossRef] [PubMed]
- IPCC. IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Zeits 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; ISBN 978-85-7035-800-4. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015; International Union of Soil Sciences: Rome, Italy, 2015. [Google Scholar]
- Adewopo, J.B.; Silveira, M.L.; Xu, S.; Gerber, S.; Sollenberger, L.E.; Martin, T.A. Management Intensification Impacts on Soil and Ecosystem Carbon Stocks in Subtropical Grasslands. Soil Sci. Am. J. 2014, 78, 977–986. [Google Scholar] [CrossRef]
- Sant-Anna, S.A.C.; Jantalia, C.P.; Sá, J.M.; Vilela, L.; Marchão, R.L.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Changes in soil organic carbon during 22 years of pastures, cropping or integrated crop/livestock systems in the Brazilian Cerrado. Nutr. Cycl. Agroecosyst. 2017, 108, 101–120. [Google Scholar] [CrossRef]
- Sisti, C.P.J.; Santos, H.P.D.; Kochhann, R.A.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M. Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in southern Brazil. Soil Tillage Res. 2004, 76, 39–58. [Google Scholar] [CrossRef]
- Urquiaga, S.; Cruz, K.H.S.; Boddey, R.M. Contribution of Nitrogen Fixation to Sugar Cane: Nitrogen-15 and Nitrogen-Balance Estimates. Soil Sci. Am. J. 1992, 56, 105–114. [Google Scholar] [CrossRef]
- Coplen, T.B. Guidelines and recommended terms for expression of stablele isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef]
- Cerri, C.C.; Feller, C.; Balesdent, J.; Victoria, R.; Plenecassegne, A. Application du traçage isotopique naturel en 13C, à l’étude de la dynamique de la matière organique dans les sols. Comptes Rendus L’Academie Sci. 1985, 300, 423–428. [Google Scholar]
- Moraes, J.F.L.; Volkoff, B.; Cerri, C.C.; Bernoux, M. Soil properties under Amazon forest and changes due to pasture installation in Rondonia, Brazil. Geoderma 1996, 70, 63–81. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods, 1st ed.; CAB International: Wallington, UK, 1985. [Google Scholar] [CrossRef]
- Cerri, C.E.P.; Easter, M.; Paustian, K.; Killian, K.; Coleman, K.; Bernoux, M.; Fallon, P.; Powlson, D.S.; Batjses, N.; Milne, E.; et al. Simulating SOC changes in 11 land use change chronosequences from the Brazilian Amazon with RothC and Century models. Agric. Ecosyst. Environ. 2007, 122, 46–57. [Google Scholar] [CrossRef]
- Lal, R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef] [PubMed]
- Eclesia, R.P.; Jobbagy, E.G.; Jackson, R.B.; Biganzoli, F.; Pineiro, G. Shifts in soil organic carbon for plantation and pasture establelishment in native forests and grasslands of South America. Glob. Chang. Biol. 2012, 18, 3237–3251. [Google Scholar] [CrossRef]
- Tuo, D.; Gao, G.; Chang, R.; Li, Z.; Ma, Y.; Wang, S.; Fu, B. Effects of revegetation and precipitation gradient on soil carbon and nitrogen variations in deep profiles on the Loess Plateau of China. Sci. Total Environ. 2018, 626, 399–441. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Carbon sequestration. Philos. Trans. R. Soc. B 2008, 363, 815–830. [Google Scholar] [CrossRef]
- Freitas, I.C.; Ribeiro, J.M.; Araújo, N.C.A.; Santos, M.V.; Sampaio, R.A.; Fernandes, L.A.; Azevedo, A.M.; Feigl, B.J.; Cerri, C.E.P.; Frazão, L.A. Agrosilvopastoral Systems and Well-Managed Pastures Increase Soil Carbon Stocks in the Brazilian Cerrado. Rangel. Ecol. Manag. 2020, 73, 776–785. [Google Scholar] [CrossRef]
- Moitinho, M.R.; Teixeira, D.D.B.; Bicalho, E.d.S.; Panosso, A.R.; Ferraudo, A.S.; Pereira, G.T.; Tsai, S.M.; Borges, B.M.F.; La Scala, N. Soil CO2 emission and soil attributes associated with the microbiota of a sugarcane area in southern Brazil. Sci. Rep. 2021, 11, 8325. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]







| System | Land Use History | |||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | |
| Forest |
|
|
|
|
| Pasture |
|
|
|
|
| Crop |
|
|
|
|
| Land Use | pH | OM | Ca | Mg | K | Al | H | V% | Sand | Silt | Clay |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cacl2 | % | mmolc dm−3 | % | ||||||||
| Forest 1 | 4.3 | 2.3 | 4.0 | 2.0 | 0.3 | 14.0 | 38.0 | 10.0 | 67 | 11 | 23.0 |
| Forest 2 | 4.0 | 2.7 | 6.0 | 2.0 | 0.4 | 13.0 | 60.0 | 11.0 | 74 | 6 | 20.0 |
| Forest 3 | 5.0 | 1.1 | 32.0 | 8.0 | 0.9 | 3.0 | 44.0 | 46.0 | 39 | 16 | 45.0 |
| Forest 4 | 4.0 | 1.3 | 7.0 | 5.0 | 1.0 | 13.0 | 98.0 | 10.0 | 12 | 12 | 30.0 |
| Pasture 1 | 4.7 | 3.4 | 33 | 8.0 | 1.3 | 1.0 | 47.0 | 47.0 | 32.0 | 19.0 | 60.0 |
| Pasture 2 | 4.8 | 1.0 | 12.0 | 4.0 | 0.4 | 0.0 | 23.0 | 42.0 | 81.0 | 4.0 | 15.0 |
| Pasture 3 | 4.9 | 1.0 | 22.0 | 6.0 | 0.3 | 0.0 | 28.0 | 52.0 | 56.0 | 7.0 | 37.0 |
| Pasture 4 | 4.8 | 2.0 | 28.0 | 7.0 | 0.5 | 1.0 | 36.0 | 49.0 | 34.0 | 8.0 | 34.0 |
| Crop 1 | 5.1 | 2.3 | 48.0 | 10.0 | 0.5 | 0.0 | 34.0 | 63.0 | 44.0 | 8.0 | 48.0 |
| Crop 2 | 4.7 | 1.3 | 17.0 | 5.0 | 0.5 | 1.0 | 26.0 | 46.0 | 78.0 | 5.0 | 18.0 |
| Crop 3 | 4.8 | 1.0 | 14.0 | 5.0 | 0.4 | 0.0 | 28.0 | 41.0 | 77.0 | 6.0 | 17.0 |
| Crop 4 | 4.2 | 1.0 | 5.0 | 3.0 | 0.3 | 4.0 | 34.0 | 18.0 | 71.0 | 6.0 | 23.0 |
| Forest | Pasture | Crop | CV 2 (%) | |
|---|---|---|---|---|
| Carbon stock (Mg C ha−1) | ||||
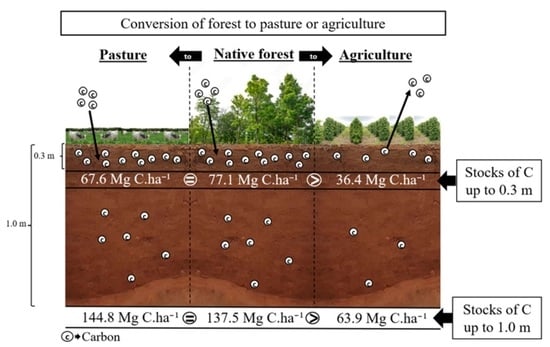
| 30 cm | 77.1 a 2 | 67.6 a | 36.4 b | 7.7 *** |
| 100 cm | 137.5 a | 144.8 a | 63.9 b | 4.2 *** |
| Nitrogen stock (Mg N ha−1) | ||||
| 30 cm | 6.3 a | 5.7 a | 3.0 b | 24.0 * |
| 100 cm | 13.8 a | 13.3 a | 6.0 b | 17.9 * |
| Forest | Crop | Pasture | Coeff. Variation 2 (%) | |
|---|---|---|---|---|
| Light fraction of soil organic matter (Mg SOM ha−1) | ||||
| 0–5 cm | 10.0 a 1 | 2.2 b | 4.0 ab | 25.2 * |
| 5–10 cm | 1.6 b | 0.9 b | 4.2 a | 35.2 ** |
| 10–20 cm | 1.8 b | 0.6 c | 3.2 a | 12.8 *** |
| 20–30 cm | 1.1 | 0.9 | 0.9 | 32.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, J.C.d.; Cardoso, A.d.S.; Lage Filho, N.M.; Faturi, C.; Silva, T.C.d.; Domingues, F.N.; Costa, V.E.; Ruggieri, A.C.; Reis, R.A.; do Rêgo, A.C. Effects of Agricultural Expansion on Soil Carbon and Nitrogen Stocks in the Amazon Deforestation Arc. Soil Syst. 2024, 8, 25. https://doi.org/10.3390/soilsystems8010025
Azevedo JCd, Cardoso AdS, Lage Filho NM, Faturi C, Silva TCd, Domingues FN, Costa VE, Ruggieri AC, Reis RA, do Rêgo AC. Effects of Agricultural Expansion on Soil Carbon and Nitrogen Stocks in the Amazon Deforestation Arc. Soil Systems. 2024; 8(1):25. https://doi.org/10.3390/soilsystems8010025
Chicago/Turabian StyleAzevedo, Jorge Cardoso de, Abmael da Silva Cardoso, Nauara Moura Lage Filho, Cristian Faturi, Thiago Carvalho da Silva, Felipe Nogueira Domingues, Vladimir Eliodoro Costa, Ana Cláudia Ruggieri, Ricardo Andrade Reis, and Aníbal Coutinho do Rêgo. 2024. "Effects of Agricultural Expansion on Soil Carbon and Nitrogen Stocks in the Amazon Deforestation Arc" Soil Systems 8, no. 1: 25. https://doi.org/10.3390/soilsystems8010025
APA StyleAzevedo, J. C. d., Cardoso, A. d. S., Lage Filho, N. M., Faturi, C., Silva, T. C. d., Domingues, F. N., Costa, V. E., Ruggieri, A. C., Reis, R. A., & do Rêgo, A. C. (2024). Effects of Agricultural Expansion on Soil Carbon and Nitrogen Stocks in the Amazon Deforestation Arc. Soil Systems, 8(1), 25. https://doi.org/10.3390/soilsystems8010025