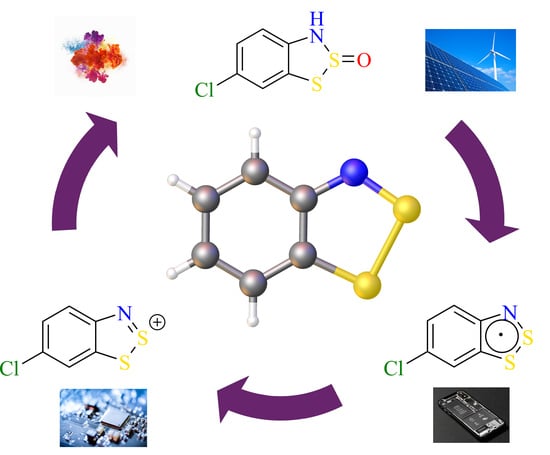
Benzo[1,2,3]dithiazole Compounds: A History of Synthesis and Their Renewed Applicability in Materials and Synthetic Chemistry, Originating from the Herz Reaction
Abstract
:1. Introduction to Benzo[1,2,3]dithiazole Compounds
1.1. Categorisation of Benzo[1,2,3]dithiazole Compounds
1.2. Formation and Characterisation of Benzo[1,2,3]dithiazole Compounds
2. Synthesis of Benzodithiazole Compounds Using the Herz Reaction
2.1. History and Contributions to Understanding and Mechanism
- (1)
- Formation of the 5-membered 1,2,3-dithiazole ring.
- (2)
- Chlorination of the carbon para to the amine (if possible) or replacement of electron-withdrawing groups that are ortho or para to the amine (Scheme 5).
- (3)
- Oxidation of the thiophenol sulphur to form the salt.
2.2. Proposed Mechanism of the Herz Reaction
2.3. Scope of Reaction
2.4. Regioselectivity
2.5. Formation of Benzo[1,2,3]dithiazole ‘Herz’ Radicals
2.6. Reactions with Multi-Fuctional Anilines
3. Applications of Benzodithiazoles as Synthetic Intermediates
3.1. Synthesis of Aminothiophenols
3.2. Formation of Benzothiazoles
3.3. Formation of Secondary 2-Aminobenzothiazoles
3.4. Formation of 2-Mercaptobenzothiazoles
3.5. Nucleophilic Aromatic Substitution
3.6. Future Applicablity in Synthesis
4. Application of the Herz Radicals as Synthetic Intermediates
4.1. Reaction with Molecular Oxygen
4.2. Synthesis of Phenazines
4.3. Synthesis of Phenothiazines
5. Applications from the Electronic Properties of Benzo[1,2,3]dithiazoles
5.1. Legacy, Visible-Light Dyes
5.2. Near-Infrared Dyes
5.3. Semiconductors
5.4. Photovoltaic or Electrochemical Cells: The Next Application?
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- Swetha, T.; Karim, M.R.; Alharbi, H.F.; Alharthi, N.H.; Bais, B.; Amin, N.; Akhtaruzzaman, M. Synthesis of New Simple Hole-Transport Materials Bearing Benzodithiazole Based Core for Perovskite Solar Cells. Sol. Energy 2019, 194, 431–435. [Google Scholar] [CrossRef]
- Baran, D.; Bryant, D.T.J.; Nicola, G.; Troughton, J.R. Radiative Heat-Blocking Materials. World Patent WO2020115663A1, 11 June 2020. [Google Scholar]
- Leckie, D.; Stephaniuk, N.T.; Arauzo, A.; Campo, J.; Rawson, J.M. Exploring Through-Bond and Through-Space Magnetic Communication in 1,3,2-Dithiazolyl Radical Complexes. Chem. Commun. 2019, 55, 9849–9852. [Google Scholar] [CrossRef] [PubMed]
- Rawson, J.M.; McManus, G.D. Benzo-Fused Dithiazolyl Radicals: From Chemical Curiosities to Materials Chemistry. Coord. Chem. Rev. 1999, 189, 135–168. [Google Scholar] [CrossRef]
- Wolmershäuser, G.; Schnauber, M.; Wilhelm, T. Benzo-1,3,2-Dithiazol-2-YL and its Derivatives: A New Class of Donor Molecules for Highly Conducting Charge Transfer Complexes. Mol. Cryst. Liq. Cryst. 1985, 120, 323–326. [Google Scholar] [CrossRef]
- Scifinder. A CAS Solution. Available online: https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf (accessed on 12 February 2021).
- Huestis, L.; Emery, I.; Steffensen, E. The Synthesis of 1,3,2-Benzothiazathiolium Salts by the Dehydration of 3 H-1,2,3-Benzodithiazole 2-Oxides. J. Heterocycl. Chem. 1966, 3, 518–520. [Google Scholar] [CrossRef]
- Bats, J.W.; Fuess, H.; Weber, K.L.; Roesky, H.W. Synthese, Struktur und einige Eigenschaften von 1,2,3-Benzodithiazolium-Salzen. Chem. Ber. 1983, 116, 1751–1755. [Google Scholar] [CrossRef]
- Huestis, L.D.; Walsh, M.L.; Hahn, N. The Herz Reaction. The Formation and Hydrolysis of Herz Compounds. J. Org. Chem. 1965, 30, 2763–2766. [Google Scholar] [CrossRef]
- Akulin, Y.I.; Gel’mont, M.M.; Strelets, B.K.; Éfros, L.S. Structures and Reactivities of Benzo-1,2,3-Dithiazolium Salts and Their Selenium Analogs. Chem. Heterocycl. Compd. 1978, 14, 733–737. [Google Scholar] [CrossRef]
- Volkova, Y.M.; Makarov, A.Y.; Zikirin, S.B.; Genaev, A.M.; Bagryanskaya, I.Y.; Zibarev, A.V. 3,1,2,4-Benzothiaselenadiazine and Related Heterocycles: Synthesis and Transformation into Herz-Type Radicals. Mendeleev Commun. 2017, 27, 19–22. [Google Scholar] [CrossRef]
- Makarov, A.Y.; Volkova, Y.M.; Shundrin, L.A.; Dmitriev, A.A.; Irtegova, I.G.; Bagryanskaya, I.Y.; Shundrina, I.K.; Gritsan, N.P.; Beckmann, J.; Zibarev, A.V. Chemistry of Herz Radicals: A New Way to Near-IR Dyes with Multiple Long-Lived and Differently-Coloured Redox States. Chem. Commun. 2020, 56, 727–730. [Google Scholar] [CrossRef]
- Strelets, B.K.; Éfros, L.S. Structure of Benzothiazolium Compounds (Herz’s Compounds) and Their Hydrolysis Products. J. Org. Chem. USSR 1969, 5, 151–154. [Google Scholar]
- Neo, A.G.; Carrillo, R.M.; Marcos, C.F. A Straightforward Synthesis of 2-Aminobenzothiazoles from Herz Compounds. Org. Biomol. Chem. 2011, 9, 4850–4855. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, W.; Aibuldinov, Y.K.; Dehaen, W. An Efficient Synthetic Route Towards Novel Thienobenzothiazoles, Thienobenzothiazepines, and Thienobenzothiazines. Tetrahedron 2013, 69, 4176–4184. [Google Scholar] [CrossRef]
- Barclay, T.M.; Cordes, A.W.; Goddard, J.D.; Mawhinney, R.C.; Oakley, R.T.; Preuss, K.E.; Reed, R.W. Benzo-Bridged Bis(1,2,3-dithiazoles) and their Selenium Analogues. Preparation, Molecular and Electronic Structures, and Redox Chemistry. J. Am. Chem. Soc. 1997, 119, 12136–12141. [Google Scholar] [CrossRef]
- Makarov, A.Y.; Chulanova, E.A.; Semenov, N.A.; Pushkarevsky, N.A.; Lonchakov, A.V.; Bogomyakov, A.S.; Irtegova, I.G.; Vasilieva, N.V.; Lork, E.; Gritsan, N.P.; et al. QITJIO: 6H-1,2,3-Benzodithiazol-6-Ylidenemalononitrile Tetrahydrofuran Solvate. Exp. Cryst. Struct. Determ. 2013, 946863. [Google Scholar] [CrossRef]
- Herz, R. Verfahren zur Darstellung von Schwefel- und Stickstoffhaltigen Kondensationsprodukten der Aromatischen Reihe. German Patent DE360690C, 6 October 1914. [Google Scholar]
- Lüttringhaus, A. Richard Herz. 1867–1936. Chem. Ber. 1956, 89, i–x. [Google Scholar] [CrossRef]
- Austad, B.C.; Rakitin, O.A. Disulfur Dichloride. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, Ltd.: Chichester, UK, 2011; Volume 1, pp. 1–9. ISBN 1915941016. [Google Scholar]
- Sawhney, S.N.; Sharma, P.; Bajaj, K.; Gupta, A. A New Synthesis of 2-Arylbenzothiazoles from 1,2,3-Benzodithiazole-2-Oxides. Synth. Commun. 1993, 23, 263–270. [Google Scholar] [CrossRef]
- Hope, P.; Wiles, L.A. The Action of Sulphur Monochloride on Aromatic Primary Amines: The Herz reaction. J. Chem. Soc. C Org. 1967, 1642–1644. [Google Scholar] [CrossRef]
- Warburton, W.K. Arylthiazathiolium Salts And o-Aminoaryl Thiols—The Herz Reaction. Chem. Rev. 1957, 57, 1011–1020. [Google Scholar] [CrossRef]
- Claus, A. Mittheilungen aus dem Universitätslaboratorium zu Freiburg i. B. Ber. der Dtsch. Chem. Ges. 1874, 7, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Sammes, M.P. Dioxazoles, Oxathiazoles and Dithiazoles. In Comprehensive Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 1984; Volume 6–7, pp. 897–946. ISBN 9780080965192. [Google Scholar]
- Claus, A. Einwirkung von Chlorschwefel auf Anilin. Ber. der Dtsch. Chem. Ges. 1870, 3, 527–528. [Google Scholar] [CrossRef] [Green Version]
- Bezzubets, M.; Rozina, V. Issledovanie v Oblasti Kislotnykh Antrakhinonnykh Soedinenii. 1. O Vliyanii Zamestitelei v Fenilaminnom Radikale Kislotnykh Antrakhinonnykh Soedinenii na Ikh Svoistva. Zhurnal Prikl. Khimii 1948, 21, 1152–1161. [Google Scholar]
- García-Valverde, M.; Torroba, T. Heterocyclic Chemistry of Sulfur Chlorides—Fast Ways to Complex Heterocycles. Eur. J. Org. Chem. 2006, 2006, 849–861. [Google Scholar] [CrossRef]
- Gompper, R.; Euchner, H.; Kast, H. Umsetzungen α.β-ungesättigter β-Amino- und β-Hydroxy-carbonylverbindungen mit Dischwefeldichlorid und verwandten Verbindungen. Justus Liebigs Ann. Chem. 1964, 675, 151–174. [Google Scholar] [CrossRef]
- Inagaki, Y.; Okazaki, R.; Inamoto, N. Chemistry of N-Thiosulfinylanilines. II. Thermolysis and Photolysis of N-Thiosulfinylanilines. Bull. Chem. Soc. Jpn. 1979, 52, 2002–2007. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, Y.; Okazaki, R.; Inamoto, N. Chemistry of N-Thiosulfinylanilines. I. Reactions of Sterically Hindered Anilines with Sulfur Chlorides. Preparation of N-Thiosulfinylanilines. Bull. Chem. Soc. Jpn. 1979, 52, 1998–2001. [Google Scholar] [CrossRef]
- Inagaki, Y.; Okazaki, R.; Inamoto, N.; Yamada, K.; Kawazura, H. Chemistry of N-Thiosulfinylanilines. III. Thiosulfinylamine—5H-1,2,3-Dithiazole Equilibrium as Studied by NMR Spectroscopy. Bull. Chem. Soc. Jpn. 1979, 52, 2008–2009. [Google Scholar] [CrossRef] [Green Version]
- Mayer, R. S,N-Compounds via Amines and Sulphur Halides. Phosphorus Sulfur Relat. Elem. 1985, 23, 277–296. [Google Scholar] [CrossRef]
- Chenard, B.L. Pyrazolothiadiazoles from 3-Aminopyrazoles: The Hetero-Herz Reaction. J. Org. Chem. 1984, 49, 1224–1227. [Google Scholar] [CrossRef]
- Iwasaki, F. The Structures of 2,4,6-Tri-tert-butyl-7,8,9-dithiazabicyclo[4.3.0]nona-1(9),2,4-triene and its 7-Oxide. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 1466–1471. [Google Scholar] [CrossRef]
- Inagaki, Y.; Okazaki, R.; Inamoto, N. Preparation of a Sterically Hindered N-Thiosulfinylaniline and its Equilibrium with a Novel Heterocycle, 5h-1,2,3-Dithiazole. Tetrahedron Lett. 1975, 16, 4575–4578. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Robson, M.J. Preparation and Properties of p-Dimethylamino-N-Thiosulphinylaniline. J. Chem. Soc. Perkin Trans. 1 1974, 1245–1247. [Google Scholar] [CrossRef]
- Okazaki, R.; Inoue, K.; Inamoto, N. Reactions of Ketone Hydrazones and β-Keto Enamines with Disulfur Dichloride. New Synthesis of Thioketones and 5H-1,2,3-Dithiazoles. Bull. Chem. Soc. Jpn. 1981, 54, 3541–3545. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, F. The Crystal and Molecular Structure of 2,4-Di-tert-butyl-6-methyl-N-thiosulphinylaniline. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 35, 2099–2103. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of Herz Salts with Malononitrile: A General Route to (6H-1,2,3-Benzodithiazol-6-ylidene)malononitriles. J. Chem. Soc. Perkin 1 2002, 3, 315–319. [Google Scholar] [CrossRef]
- Mayer, R.; Domschke, G.; Bleisch, S.; Bartl, A. 1,2-Thiazet-2-yle, eine Neue Klasse Stabiler Radikale. Tetrahedron Lett. 1978, 19, 4003–4006. [Google Scholar] [CrossRef]
- Fabian, J.; Mayer, R.; Bleisch, S.; Zahradník, R. Electronic Excitation of Organosulfur Radicals. Phosphorus Sulfur Relat. Elem. 1982, 13, 107–117. [Google Scholar] [CrossRef]
- Bartl, A.; Siegfried, B.; Guenter, D.; Mayer, R. Verfahren Zur Erzeugung Persistenter(Stabiler)1,2,3-Dithiazolyle. German Patent DD156183A1, 4 August 1981. [Google Scholar]
- Yu, X.; Mailman, A.; Lekin, K.; Assoud, A.; Dube, P.A.; Oakley, R.T. A Bimodal Oxobenzene-Bridged Bisdithiazolyl Radical Conductor. Cryst. Growth Des. 2012, 12, 2485–2494. [Google Scholar] [CrossRef]
- Chulanova, E.A.; Irtegova, I.G.; Vasilieva, N.V.; Bagryanskaya, I.Y.; Gritsan, N.P.; Zibarev, A.V. Novel Long-Lived N-Heterocyclic Radical Anion: A Hybrid of 1,2,5-Thiadiazo- and 1,2,3-Dithiazolidyls. Mendeleev Commun. 2015, 25, 336–338. [Google Scholar] [CrossRef]
- Parikh, K.; Warren, C.; Caracio, R. Education on Reactive Oxygen Species to Lay the Foundation for Understanding Therapeutic Advances in Anticancer Therapy. J. Clin. Oncol. 2021, 39, 478. [Google Scholar] [CrossRef]
- Makarov, A.Y.; Chulanova, E.A.; Semenov, N.A.; Pushkarevsky, N.A.; Lonchakov, A.V.; Bogomyakov, A.S.; Irtegova, I.G.; Vasilieva, N.V.; Lork, E.; Gritsan, N.P.; et al. A Novel Sulfur-Nitrogen π-Heterocyclic Radical Anion, (6H-1,2,3-Benzodithiazol-6-ylidene)malononitrilidyl, and its Homo- and Heterospin Salts. Polyhedron 2014, 72, 43–49. [Google Scholar] [CrossRef]
- Lonchakov, A.V.; Rakitin, O.A.; Gritsan, N.P.; Zibarev, A.V. Breathing Some New Life into an Old Topic: Chalcogen-Nitrogen π-Heterocycles as Electron Acceptors. Molecules 2013, 18, 9850–9900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ried, W.; Valentin, J.; Schubert, M. The Herz Reaction with 1,5-Diaminonaphthalene. Angew. Chem. Internat. Ed. 1965, 4, 711. [Google Scholar]
- Makarov, A.Y.; Zhivonitko, V.V.; Makarov, A.G.; Zikirin, S.B.; Bagryanskaya, I.Y.; Bagryansky, V.A.; Gatilov, Y.V.; Irtegova, I.G.; Shakirov, M.M.; Zibarev, A.V. Interaction of 1,3,2,4-benzodithiadiazines and their 1-Se congeners with Ph3P and some properties of the iminophosphorane products. Inorg. Chem. 2011, 50, 3017–3027. [Google Scholar] [CrossRef]
- Makarov, A.Y.; Zhivonitko, V.V.; Makarov, A.G.; Zikirin, S.B.; Bagryanskaya, I.Y.; Bagryansky, V.A.; Gatilov, Y.V.; Irtegova, I.G.; Shakirov, M.M.; Zibarev, A.V. EWADAI: Benzo[1,2-c:3,4-c’:5,6-c’’]tris[1,2,5]thiadiazole. Exp. Cryst. Struct. Determ. 2011, 769835. [Google Scholar] [CrossRef]
- Maier, G.; Schrot, J.; Reisenauer, H.P.; Janoschek, R. C5S2 (1,2,3,4-Pentatetraen-1,5-dithion), ein neues Sulfid des Kohlenstoffs. Chem. Ber. 1990, 123, 1753–1756. [Google Scholar] [CrossRef]
- Barclay, T.M.; Burgess, I.J.; Cordes, A.W.; Oakley, R.T.; Reed, R.W. Preparation and Structural Characterization of Naphtho[2,1-d:6,5-d′]bis([1,2,3]dithiazole) NT and π-Stacked Mixed Valence Salt [NT]3[BF4]2. Chem. Commun. 1998, 1939–1940. [Google Scholar] [CrossRef]
- Mailman, A.; Leitch, A.A.; Yong, W.; Steven, E.; Winter, S.M.; Claridge, R.C.M.; Assoud, A.; Tse, J.S.; Desgreniers, S.; Secco, R.A.; et al. The Power of Packing: Metallization of an Organic Semiconductor. J. Am. Chem. Soc. 2017, 139, 2180–2183. [Google Scholar] [CrossRef]
- Beer, L.; Britten, J.F.; Brusso, J.L.; Cordes, A.W.; Haddon, R.C.; Itkis, M.E.; MacGregor, D.S.; Oakley, R.T.; Reed, R.W.; Robertson, C.M. Prototypal Dithiazolodithiazolyl Radicals: Synthesis, Structures, and Transport Properties. J. Am. Chem. Soc. 2003, 125, 14394–14403. [Google Scholar] [CrossRef]
- Röder, L.; Nicholls, A.J.; Baxendale, I.R. Flow Hydrodediazoniation of Aromatic Heterocycles. Molecules 2019, 24, 1996. [Google Scholar] [CrossRef] [Green Version]
- Reitzenstein, F. Verfahren zur Darstellung von Azoxyverbindungen. J. Für Prakt. Chem. 1910, 82, 252–270. [Google Scholar] [CrossRef] [Green Version]
- Polo, C.; Ramos, V.; Torroba, T.; Rakitin, O.A.; Rees, C.W. One Pot Synthesis of 1,2,3,-Benzodithiazol-6-ones. Tetrahedron 1998, 54, 223–232. [Google Scholar] [CrossRef]
- Mayer, R.; Gúnter, D.; Bleisch, S.; Fabian, J.; Bartl, A.; Stasko, A. 1,2,3-Dithiazolyle, Eine Neue Klasse Persistenter Radikale. Collect. Czechoslov. Chem. Commun. 1984, 49, 684–703. [Google Scholar] [CrossRef]
- Belica, P.S.; Manchand, P.S. A Convenient Synthesis of 2-Aminonaphthalene-1-thiol. Synthesis 1990, 1990, 539–540. [Google Scholar] [CrossRef]
- Merck. Available online: https://www.sigmaaldrich.com/catalog/search/substructure/SubstructureSearchResultsPage (accessed on 5 February 2021).
- Wang, P.; Zhao, S.; Liu, Z.; Cui, P.; Liang, Z. A Green Industrialized Preparation Method of Rubber Peptizer DBD. Chinese Patent CN 104774165, 16 March 2015. [Google Scholar]
- Mayuri, P.; Kumar, K.; Goud, N.S. Achaiah Review on 2-Substituted Benzothiazole: Diversity of Synthestic Methods and Biological Activities. Int. J. Pharm. Sci. Res. 2015, 7, 1375–1385. [Google Scholar]
- Hojo, M.; Takagi, Y.; Ogata, Y. Kinetics of the Reduction of Nitrobenzenes by Sodium Disulfide. J. Am. Chem. Soc. 1960, 82, 2459–2462. [Google Scholar] [CrossRef]
- Huber, D.; Andermann, G.; Leclerc, G. Selective Reduction of Aromatic/Aliphatic Nitro Groups by Sodium Sulfide. Tetrahedron Lett. 1988, 29, 635–638. [Google Scholar] [CrossRef]
- McLaughlin, M.A.; Barnes, D.M. A Practical and Selective Reduction of Nitroarenes using Elemental Sulfur and Mild Base. Tetrahedron Lett. 2006, 47, 9095–9097. [Google Scholar] [CrossRef]
- Pradhan, N.C.; Sharma, M.M. Reactions of Nitrochlorobenzenes with Sodium Sulfide: Change in Selectivity with Phase-Transfer Catalysts. Ind. Eng. Chem. Res. 1992, 31, 1606–1609. [Google Scholar] [CrossRef]
- Nicholls, A.J. Investigation of New Routes to Benzothiazole or 2-Aminothiophenol as Feedstocks for the Synthesis of PeptonTM. Ph.D. Thesis, University of Durham, Durham, UK, 2021. [Google Scholar]
- Rajeswari, T.; Rekha, T.; Dinneswara Reddy, G.; Padmaja, A.; Padmavathi, V. Synthesis and Antibacterial Activity of Benzazolyl Azolyl Sulfamoyl Acetamides. J. Heterocycl. Chem. 2019, 56, 2449–2459. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Z. Carbapenem Derivatives Containing Formamide Heterocyclic Mercaptopyrrolidine. Chinese Patent CN 101613352A, 27 March 2009. [Google Scholar]
- Piscitelli, F.; Ballatore, C.; Smith, A.B. Solid Phase Synthesis of 2-Aminobenzothiazoles. Bioorg. Med. Chem. Lett. 2010, 20, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Recent Trends in the Chemistry of 2-Aminobenzothiazoles. J. Sulfur Chem. 2009, 30, 74–107. [Google Scholar] [CrossRef]
- Dadmal, T.L.; Katre, S.D.; Mandewale, M.C.; Kumbhare, R.M. Contemporary Progress in the Synthesis and Reactions of 2-Aminobenzothiazole: A Review. New J. Chem. 2018, 42, 776–797. [Google Scholar] [CrossRef]
- Ulrich, H. Product Class 18: Benzothiazoles and Related Compounds. In Category 2, Hetarenes and Related Ring Systems; Schaumann, Ed.; Georg Thieme Verlag: Stuttgart, Germany, 2002. [Google Scholar]
- Leaper, J.M.F. Some Heterocyclic Derivatives Of Diphenyl. J. Am. Chem. Soc. 1931, 53, 1891–1896. [Google Scholar] [CrossRef]
- Herz, R. Verfahren Zur Herstellung von schwefel- und stickstoffhaltigen Kondensationprodukten der aromatischen Reihe. German Patent DE487849C, 5 December 1929. [Google Scholar]
- Booth, G. Nitro Compounds, Aromatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Volume 24, pp. 392–448. ISBN 9783924063825. [Google Scholar]
- Strelets, B.K.; Éfros, L.S.; Akulin, Y.I. Reaction of Benzo-1,2,3-Dithiazolium Salts with Aromatic Amines. Chem. Heterocycl. Compd. 1976, 12, 161–163. [Google Scholar] [CrossRef]
- Naitoh, T.; Horie, T.; Nagato, S.; Kagaya, T.; Kubota, A.; Akasaka, K. Application of Pharmacokinetic Studies to a Novel Antidepressant, E2011. Xenobiotica 1994, 24, 819–826. [Google Scholar] [CrossRef]
- Sheldon, L.J. Combination Therapy for Dementia, Depression and Apathy. U.S. Patent 8,703,764, 22 April 2014. [Google Scholar]
- Sciences, B. NVP-231—CAS 362003-83-6. Available online: https://www.bocsci.com/nvp-231-cas-362003-83-6-item-84-272254.html?msclkid=7f9d3e1270781ed1eb4f5e3740f151e7&utm_source=bing&utm_medium=cpc&utm_campaign=Bocsci-Inhibitor&utm_term=362003-83-6&utm_content=362003-83-6 (accessed on 11 February 2021).
- Nakamura, H.; Murayama, T. Pharmaceutical Composition For Preventing Or Treating Niemann-Pick Disease Type C. Japanese Patent JP2017071576A, 13 April 2017. [Google Scholar]
- Fathman, G.C.; Soares, L.R. Combination Of Low Dose Il-2 And An Inhibitor Of Treg Il-2r Desensitization To Treat Autoimmune And Allergic Inflammatory Diseases. World Patent WO2018218119A1, 25 May 2018. [Google Scholar]
- Avelar, R.; Maiti, A.; Tolekis, P.M.; Cashman, J.D.; Gravett, D.M. Sutures and Anti-Scarring Agents. World Patent WO2007089878A2, 9 August 2007. [Google Scholar]
- Johnson, J.A.; Pi, Z.; Qiao, J.X.; Kim, S.-H.; Wang, T.C.; Jiang, J.; Finlay, H.; Lloyd, J. Sulfone Amide Linked Benzothiazole Inhibitors Of Endothelial Lipase. U.S. Patent 10,173,991, 8 January 2019. [Google Scholar]
- Makarov, A.Y.; Kim, S.N.; Gritsan, N.P.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Zibarev, A.V. Interaction of 1,2,3-benzodithiazolyls (Herz radicals) with dioxygen. Mendeleev Commun. 2005, 15, 14–17. [Google Scholar] [CrossRef]
- Mortzfeld, F.B.; Pietruszka, J.; Baxendale, I.R. A Simple and Efficient Flow Preparation of Pyocyanin a Virulence Factor of Pseudomonas aeruginosa. Eur. J. Org. Chem. 2019, 2019, 5424–5433. [Google Scholar] [CrossRef]
- Kommi, D.N.; Jadhavar, P.S.; Kumar, D.; Chakraborti, A.K. “All-water” One-Pot Diverse Synthesis of 1,2-Disubstituted Benzimidazoles: Hydrogen Bond Driven ‘Synergistic Electrophile-Nucleophile Dual Activation’ by Water. Green Chem. 2013, 15, 798–810. [Google Scholar] [CrossRef]
- Nicholls, A.J.; Barber, T.; Baxendale, I.R. The Synthesis and Utility of Metal-Nitrosophenolato Compounds—Highlighting the Baudisch Reaction. Molecules 2019, 24, 4018. [Google Scholar] [CrossRef] [Green Version]
- Creencia, E.C.; Kosaka, M.; Muramatsu, T.; Kobayashi, M.; Iizuka, T.; Horaguchi, T. Microwave-Assisted Cadogan Reaction for the Synthesis of 2-Aryl-2 H -indazoles, 2-Aryl-1 H -Benzimidazoles, 2-Carbonylindoles, Carbazole, and Phenazine. J. Heterocycl. Chem. 2009, 46, 1309–1317. [Google Scholar] [CrossRef]
- Benati, L.; Montevecchi, P.C.; Spagnolo, P. Benzenesulphenanilidyl Radicals. Reactivity of 4′-Methoxy- and 4′-Methoxy-2-nitro-Benzenesulphenanilidyl Radicals. J. Chem. Soc. Perkin Trans. 1 1982, 3049–3053. [Google Scholar] [CrossRef]
- Zhivonitko, V.V.; Makarov, A.Y.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Shakirov, M.M.; Zibarev, A.V. New Polysulfur-Nitrogen Heterocycles by Thermolysis of 1, 3λ4δ2,2,4-Benzodithiadiazines in the Hydrocarbon and Fluorocarbon Series. Eur. J. Inorg. Chem. 2005, 4099–4108. [Google Scholar] [CrossRef]
- Zhivonitko, V.V.; Makarov, A.Y.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Shakirov, M.M.; Zibarev, A.V. MAVQUW: 4,6,7,8,9,11-Hexafluoro(1,2,3)dithiazolo(5,4-b)phenothiazine. Exp. Cryst. Struct. Determ. 2006, 268802. [Google Scholar] [CrossRef]
- Zhivonitko, V.V.; Makarov, A.Y.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Shakirov, M.M.; Zibarev, A.V. MAVQOQ: 4,5,7,8,9,10-Hexafluoro(1,2,3)dithiazolo(4,5-c)phenothiazine. Exp. Cryst. Struct. Determ. 2006, 268801. [Google Scholar] [CrossRef]
- Smithson, C.S.; Macdonald, D.J.; Letvenuk, T.M.; Carello, C.E.; Jennings, M.; Lough, A.J.; Britten, J.; Preuss, K.E. A 1,2,3-Dithiazolyl-o-Naphthoquinone: A Neutral Radical with Isolable Cation and Anion Oxidation States. Dalt. Trans. 2016, 45, 9608–9620. [Google Scholar] [CrossRef]
- Herz, R.; Brunner, W. Verfahren zur Darsellung von rosa bis rot farbenden Kupenfarbstoffen. German Patent DE525668 (C), 7 September 1931. [Google Scholar]
- Herz, R. Verfahren zur Herstellung von Kuepenfarbstoffen. German Patent DE398877C, 29 June 1916. [Google Scholar]
- Barclay, T.M.; Cordes, A.W.; Oakley, R.T.; Preuss, K.E.; Reed, R.W. Charge Transfer Chemistry of Benzo[2,1- c:3,4- c ‘ ]bis(1,2,3-dithiazole) (BT). Preparation and Structural Characterization of [BT][ClO 4 ] and [BT] 3 [X] 2 (X = ClO 4 - and FSO 3 - ). Chem. Mater. 1999, 11, 164–169. [Google Scholar] [CrossRef]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef]
- Barbieri, A.; Bandini, E.; Monti, F.; Praveen, V.K.; Armaroli, N. The Rise of Near-Infrared Emitters: Organic Dyes, Porphyrinoids, and Transition Metal Complexes. Top. Curr. Chem. 2016, 374, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Nowak, M.J.; Dormann, E.; Wudl, F. Organic Conductors Based on Diradicals: The Benzobisdithiazole (BBDT) System. Synth. Met. 1986, 14, 233–238. [Google Scholar] [CrossRef]
- Dormann, E.; Nowak, M.J.; Williams, K.A.; Angus, R.O.; Wudl, F. Benzobisdithiazole (BBDT): An Electron Spin Resonance Study. J. Am. Chem. Soc. 1987, 109, 2594–2599. [Google Scholar] [CrossRef]
- Fujita, W.; Awaga, K. Organic Ferromagnetism of Tc = 6.7 K Driven by Evaporation of Crystal Solvent. Chem. Phys. Lett. 2002, 357, 385–388. [Google Scholar] [CrossRef]
- Bryce, M.R. Recent Progress on Conducting Organic Charge-Transfer Salts. Chem. Soc. Rev. 1991, 20, 355–390. [Google Scholar] [CrossRef]
- Genin, H.; Hoffmann, R. Theoretical Tinkering: The Search for Magnetically Ordered Organic Polymers Built From Sulfur, Carbon, Nitrogen-Containing Five-Membered Rings. Macromolecules 1998, 31, 444–455. [Google Scholar] [CrossRef]
- Lonchakov, A.V. Molecular Design of Precursors of the Chalcogen-Nitrogen Heterocyclic Radical Anions and Theoretical Analysis of the Magnetic Properties of their Salts; Russian Academy of Science: Novosibirsk, Russia, 2013. [Google Scholar]
- Mailman, A.; Leitch, A.A.; Yong, W.; Steven, E.; Winter, S.M.; Claridge, R.C.M.; Assoud, A.; Tse, J.S.; Desgreniers, S.; Secco, R.A.; et al. 4,8-dioxo-4,8-dihydrobenzo[1,2-d:5,4-d’]bis[1,2,3]dithiazol-6-ium-3-ide. Exp. Cryst. Struct. Determ. 2017, 1566903. [Google Scholar] [CrossRef]
- Lekin, K.; Leitch, A.A.; Assoud, A.; Yong, W.; Desmarais, J.; Tse, J.S.; Desgreniers, S.; Secco, R.A.; Oakley, R.T. Benzoquinone-Bridged Heterocyclic Zwitterions as Building Blocks for Molecular Semiconductors and Metals. Inorg. Chem. 2018, 57, 4757–4770. [Google Scholar] [CrossRef]
- Lekin, K.; Leitch, A.A.; Assoud, A.; Yong, W.; Desmarais, J.; Tse, J.S.; Desgreniers, S.; Secco, R.A.; Oakley, R.T. FIDYUP: 4,8-dioxo-4,8-dihydrobenzo[1,2-d:5,4-d’]bis[1,2,3]thiaselenazol-6-ium-3-ide. Exp. Cryst. Struct. Determ. 2018, 1825118. [Google Scholar] [CrossRef]
- Lekin, K.; Leitch, A.A.; Assoud, A.; Yong, W.; Desmarais, J.; Tse, J.S.; Desgreniers, S.; Secco, R.A.; Oakley, R.T. FIDYUP01: 4,8-dioxo-4,8-dihydrobenzo[1,2-d:5,4-d’]bis[1,2,3]thiaselenazol-6-ium-3-ide. Exp. Cryst. Struct. Determ. 2018, 1825122. [Google Scholar] [CrossRef]
- Mailman, A.; Robertson, C.M.; Winter, S.M.; Dube, P.A.; Oakley, R.T. The Importance of Electronic Dimensionality in Multiorbital Radical Conductors. Inorg. Chem. 2019, 58, 6495–6506. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Mailman, A.; Lekin, K.; Assoud, A.; Robertson, C.M.; Noll, B.C.; Campana, C.F.; Howard, J.A.K.; Dube, P.A.; Oakley, R.T. Semiquinone-Bridged Bisdithiazolyl Radicals as Neutral Radical Conductors. J. Am. Chem. Soc. 2012, 134, 2264–2275. [Google Scholar] [CrossRef]
- Mailman, A.; Winter, S.M.; Yu, X.; Robertson, C.M.; Yong, W.; Tse, J.S.; Secco, R.A.; Liu, Z.; Dube, P.A.; Howard, J.A.K.; et al. Crossing the Insulator-to-Metal Barrier with a Thiazyl Radical Conductor. J. Am. Chem. Soc. 2012, 134, 9886–9889. [Google Scholar] [CrossRef]
- Yu, X.; Mailman, A.; Dube, P.A.; Assoud, A.; Oakley, R.T. First Semiquinone-Bridged Bisdithiazolyl Radical Conductor: A Canted Antiferromagnet Displaying a Spin-Flop Transition. Chem. Commun. 2011, 47, 4655–4657. [Google Scholar] [CrossRef] [PubMed]
- Mailman, A.; Wong, J.W.L.; Winter, S.M.; Claridge, R.C.M.; Robertson, C.M.; Assoud, A.; Yong, W.; Steven, E.; Dube, P.A.; Tse, J.S.; et al. Fine Tuning the Performance of Multiorbital Radical Conductors by Substituent Effects. J. Am. Chem. Soc. 2017, 139, 1625–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Wang, Y.; You, H.; Lee, W.; Michinobu, T.; Kim, B.J. Impact of Incorporating Nitrogen Atoms in Naphthalenediimide-Based Polymer Acceptors on the Charge Generation, Device Performance, and Stability of All-Polymer Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 35896–35903. [Google Scholar] [CrossRef]
- Tuttle, M.R.; Zhang, S. Bisthiazolyl Quinones: Stabilizing Organic Electrode Materials with Sulfur-Rich Thiazyl Motifs. Chem. Mater. 2020, 32, 255–261. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]









































 |
 |
 |
 |
 |
 |
 |
| Solid Formula | Measured Conductivity/S cm−1 (One Significant Figure) |
|---|---|
 28a | 10−5 |
 28b | 10−2 |
 28c | 10−5 |
 28d | 10−2 |
 28e | 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholls, A.J.; Baxendale, I.R. Benzo[1,2,3]dithiazole Compounds: A History of Synthesis and Their Renewed Applicability in Materials and Synthetic Chemistry, Originating from the Herz Reaction. Reactions 2021, 2, 175-208. https://doi.org/10.3390/reactions2030013
Nicholls AJ, Baxendale IR. Benzo[1,2,3]dithiazole Compounds: A History of Synthesis and Their Renewed Applicability in Materials and Synthetic Chemistry, Originating from the Herz Reaction. Reactions. 2021; 2(3):175-208. https://doi.org/10.3390/reactions2030013
Chicago/Turabian StyleNicholls, Alexander J., and Ian R. Baxendale. 2021. "Benzo[1,2,3]dithiazole Compounds: A History of Synthesis and Their Renewed Applicability in Materials and Synthetic Chemistry, Originating from the Herz Reaction" Reactions 2, no. 3: 175-208. https://doi.org/10.3390/reactions2030013
APA StyleNicholls, A. J., & Baxendale, I. R. (2021). Benzo[1,2,3]dithiazole Compounds: A History of Synthesis and Their Renewed Applicability in Materials and Synthetic Chemistry, Originating from the Herz Reaction. Reactions, 2(3), 175-208. https://doi.org/10.3390/reactions2030013