4. Experimental Section
All reagents were purchased from commercial sources and used without further purification. 1H and 13C NMR spectra were recorded with Bruker DPX-400 and JEOL JMN-ECZ400S spectrometers (400 MHz and 100 MHz, respectively) using TMS as an internal standard. The assignments of the 13C NMR spectra were performed with DEPT experiments. IR spectra were recorded with a JASCO FT/IR-4200 spectrometer equipped with an ATM detector. High-resolution mass spectra were obtained with an AB SCEIX Triplet TOF 4600 mass spectrometer. Microwave heating was performed with an Anton Paar Microwave 300 (850 W, 2455 MHz) and an Anton Paar Microwave 400 (850 W, 2450 MHz) using a 10 mL glass vessel. Diffraction data were collected at 93 K under a cold N2 gas stream with a Rigaku XtaLAB Synergy-S/Mo system (λ = 0.71073 Å (Mo-Kα)). The integrated data were analyzed by using a Yadokari-XG software package. The structures were solved with the ShelXT structure solution program using intrinsic phasing and refined with the ShelXL refinement package using least-squares minimization. Anisotropic refinement was performed for all non-hydrogen atoms, and all the hydrogen atoms were put in calculated positions.
Synthesis of α-nitrocinnamate 1a. α-Nitrocinnamate 1 was synthesized using a somewhat modified method previously described in the literature. Aniline (0.91 mL, 10.0 mmol) and magnesium sulfate (400 mg) were added to a solution of 4-methylbenzaldehyde (10.0 mmol) in THF (5 mL), and the mixture was stirred at room temperature for 5 h. After filtrations of magnesium sulfate, the filtrate was concentrated under reduced pressure to afford imine (2.0 g, 9.7 mmol, 97%) as a brown solid, which was used for the next step without further purification. A solution of imine (2.0 g, 9.7 mmol) and nitroacetate (1.08 mL, 9.7 mmol, CAS No. 626-35-7) in acetic anhydride (5 mL) was heated at 60 °C for 18 h. The reaction mixture was poured into water (100 mL) and extracted with dichloromethane (50 mL × 3). Organic layers were dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The residue was treated with flash column chromatography on silica gel (hexane/ethyl acetate = 9/1) to afford α-nitrocinnamate 1a (1520 mg, 6.5 mmol, 65%) as a yellow oil. When the aldehyde could not be completely separated, distillation was performed to remove it. The recrystallization of the product using hexane/chloroform afforded (Z) isomer. Other cinnamates 1b–f, 4, and 5 were synthesized in the same way.
Ethyl 3-(4-methylphenyl)-2-nitropropenoate (1a) [
26]. Yellow plates.
1H NMR (400 MHz, CDCl
3) δ 7.50 (s, 1H), 7.32 (d,
J = 8.2 Hz, 2H), 7.22 (d,
J = 8.2 Hz, 2H), 4.37 (q,
J = 7.3 Hz, 2H), 2.39 (s, 3H) 1.36 (t,
J = 7.3 Hz, 3H).
Ethyl 3-(4-methylphenyl)-2-nitropropenoate (1b) [
27]. Brown plates.
1H NMR (400 MHz, CDCl
3) δ 7.46 (s, 1H), 7.39 (d,
J = 8.8 Hz, 2H), 6.92 (d,
J = 8.2 Hz, 2H), 4.36 (q,
J = 7.3 Hz, 2H), 3.85 (s, 3H), 1.36 (t,
J = 7.3 Hz, 3H).
Ethyl 2-nitro-3-phenylpropenoate (1c) [
26]. Yellow plates.
1H NMR (400 MHz, CDCl
3) δ 7.59 (s, 1H), 7.40–7.51 (m, 5H), 4.39 (q,
J = 6.9 Hz, 2H), 1.37 (t,
J = 6.9 Hz, 3H).
Ethyl 3-(4-bromophenyl)-2-nitropropenoate (1d) [
26]. Yellow plates.
1H NMR (400 MHz, CDCl
3) δ 7.57 (d,
J = 8.5 Hz, 2H), 7.47 (s, 1H), 7.28 (d,
J = 8.5 Hz, 2H), 4.29 (q,
J = 7.3 Hz, 2H), 1.37 (t,
J = 7.3 Hz, 3H).
Ethyl 3-[(4-trifluoromethyl)phenyl]-2-nitropropenoate (1e) [
26]. Yellow plates.
1H NMR (400 MHz, CDCl
3) δ 7.69 (d,
J = 8.3 Hz, 2H), 7.50 (s, 1H), 7.53 (d,
J = 8.3 Hz, 2H), 4.05 (q,
J = 7.0 Hz, 2H), 1.38 (t,
J = 7.0 Hz, 3H).
Ethyl 2-nitro-3-(4-nitrophenyl)propenoate (1f) [
27]. White solid.
1H NMR (400 MHz, CDCl
3) δ 8.28 (d,
J = 8.7 Hz, 2H), 7.60 (s, 1H), 7.59 (d,
J = 8.7 Hz, 2H), 4.42 (q,
J = 6.9 Hz, 2H), 1.39 (t,
J = 6.9 Hz, 3H).
Ethyl 2-ethanoyl-3-(4-methylphenyl)propenoate (4) [
28]. Colorless plates.
1H NMR (400 MHz, CDCl
3) δ 7.63 (s, 1H), 7.29 (d,
J = 8.3 Hz, 2H), 7.17 (d,
J = 8.3 Hz, 2H), 4.29 (q,
J = 7.0 Hz, 2H), 2.36 (s, 3H), 2.35 (s, 3H), 1.32 (t,
J = 7.0 Hz, 3H).
Ethyl 2-cyano-3-(4-methylphenyl)propenoate (5) [
29]. Colorless needles.
1H NMR (400 MHz, CDCl
3) δ 8.23 (s, 1H), 7.91 (d,
J = 8.0 Hz, 2H), 7.31 (d,
J = 8.0 Hz, 2H), 3.93 (s, 3H), 2.44 (s, 3H).
Diels–Alder reaction of α-nitrocinnamate. 2,3-dimethyl-1,3-butadiene 2 (0.28 mL, 2.5 mmol) was added to a solution of α-nitrocinnamate 1a (117 mg, 0.5 mmol) in MeCN (1 mL), and the resultant solution was heated at 180 °C for 2 h under microwave irradiation. After the removal of the solvent under reduced pressure, the residue was subjected to flash column chromatography on silica gel (hexane/ethyl acetate = 95/5) to afford cycloadduct 3a (151 mg, 0.475 mmol, 95%) as a pale-yellow oil. When other substrates were used or conditions were changed, the reaction was conducted in the same way.
4-Ethoxycarbonyl-1,2-dimethyl-5-(4-methylphenyl)-4-nitrocyclohexene (3a). Pale-yellow oil (dr = 56/44). Major isomer: 1H NMR (400 MHz, CDCl3) δ 6.9–7.9 (m, 4H), 4.07 (q, J = 7.2 Hz, 2H), 4.05 (d, J = 8.0 Hz, 1H), 2.7–3.0 (m, 3H), 2.30 (s, 3H), 2.28 (br d, J = 18.8 Hz, 1H), 1.72 (s, 3H), 1.68 (s, 3H), 1.16 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 165.9 (C), 137.6 (C), 137.1 (C), 129.5 (CH), 128.4 (CH), 125.7 (C), 121.2 (C), 95.7 (C), 62.7 (CH2), 42.8 (CH), 36.2 (CH2), 34.0 (CH2), 21.2 (CH3), 19.2 (CH3), 18.6 (CH3), 13.8 (CH3). Minor isomer: 1H NMR (400 MHz, CDCl3) δ 6.9–7.9 (m, 4H), 4.24 (dq, J = 7.2, 14.4 Hz, 1H), 4.21 (dq, J = 7.2, 14.4 Hz, 1H), 3.95 (d, J = 7.6 Hz, 1H), 2.7–3.0 (m, 3H), 2.39 (br d, J = 17.2 Hz, 1H), 2.30 (s, 3H), 1.74 (s, 3H), 1.69 (s, 3H), 1.22 (dd, J = 7.2, 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 166.2 (C), 137.6 (C), 136.5 (C), 129.4 (CH), 128.4 (CH), 126.4 (C), 121.2 (C), 95.3 (C), 62.8 (CH2), 43.8 (CH), 37.7 (CH2), 33.9 (CH2), 21.2 (CH3), 19.4 (CH3), 18.4 (CH3), 13.8 (CH3). IR (KBr/cm−1) 1753, 1553; HRMS (ESI/TOF) calculated for (M + H+) C18H24NO4: 318.1700, found: 318.1696.
4-Ethoxycarbonyl-5-(4-methoxyphenyl)-1,2-dimethyl-4-nitrocyclohexene (3b). Pale-yellow oil (dr = 70/30). Major isomer: 1H NMR (400 MHz, CDCl3) δ 7.07 (d, J = 8.8 Hz, 2H), 6.79 (d, J = 8.8 Hz, 2H), 4.11 (q, J = 7.2 Hz, 2H), 4.05 (d, J = 7.6 Hz, 1H), 3.77 (s, 3H), 2.7–3.1 (m, 3H), 2.27 (br d, J = 18.4 Hz, 1H), 1.72 (s, 3H), 1.67 (s, 3H), 1.56 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 165.9 (C), 159.2 (C), 132.1 (C), 129.6 (CH), 125.7 (C), 121.2 (C), 114.2 (CH), 95.6 (C), 62.7 (CH2), 55.3 (CH3), 42.4 (CH), 36.3 (CH2), 34.0 (CH2), 19.2 (CH3), 18.6 (CH3), 13.9 (CH3). Minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.02 (d, J = 8.8 Hz, 2H), 6.79 (d, J = 8.8 Hz, 2H), 4.23 (dq, J = 7.2, 14.4 Hz, 1H),4.22 (dq, J = 7.2, 14.4 Hz, 1H), 3.93 (d, J = 7.2 Hz, 1H), 3.77 (s, 3H), 2.7–3.1 (m, 3H), 2.27 (br d, J = 17.2 Hz, 1H), 1.72 (s, 3H), 1.67 (s, 3H), 1.22 (dd, J = 7.2, 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 166.2 (C), 159.2 (C), 131.5 (C), 129.6 (CH), 125.7 (C), 121.2 (C), 114.0 (CH), 95.3 (C), 62.7 (CH2), 55.3 (CH3), 43.5 (CH), 37.8 (CH2), 34.0 (CH2), 19.4 (CH3), 18.4 (CH3), 13.9 (CH3); HRMS (ESI/TOF) calculated for (M + H+) C18H24NO5: 334.1649, found: 334.1648.
4-Ethoxycarbonyl-1,2-dimethyl-4-nitro-5-phenylcyclohexene (3c). Pale-yellow oil (dr = 62/38). Major isomer: 1H NMR (400 MHz, CDCl3) δ 7.28–7.10 (m, 5H), 4.08 (br d, J = 7.3 Hz, 1H), 4.05 (q, J = 7.3 Hz, 2H), 2.72–3.00 (m, 3H), 2.30 (br d, J = 18.3 Hz, 1H), 1.68 (s, 3H), 1.72 (s, 3H), 1.12 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 165.9 (C), 140.2 (C), 128.8 (CH), 128.5 (CH), 127.9 (CH), 125.7 (C), 121.3 (C), 95.6 (C), 62.7 (CH2), 43.1 (CH), 36.1 (CH2), 33.9 (CH2), 19.3 (CH3), 18.6 (CH3), 13.8 (CH3). Minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.28–7.10 (m, 5H), 4.22 (dq, J = 7.3, 10.6 Hz, 1H), 4.21 (dq, J = 7.3, 10.6 Hz, 1H), 3.97 (br dd, J = 7.8, 2.3 Hz, 1H), 2.72–3.00 (m, 3H), 2.41 (br d, J = 17.4 Hz, 1H), 1.73 (s, 3H), 1.69 (s, 3H), 1.12 (dd, J = 7.3, 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 166.2 (C), 139.5 (C), 128.7 (CH), 128.5 (CH), 127.9 (CH), 126.4 (C), 121.2 (C), 95.2 (C), 62.9 (CH2), 44.2 (CH), 37.6 (CH2), 34.0 (CH2), 19.5 (CH3), 18.4 (CH3), 13.8 (CH3); HRMS (ESI/TOF) calculated for (M + H+) C17H22NO4: 304.1543, found: 304.1540.
5-(4-Bromophenyl)-4-ethoxycarbonyl-1,2-dimethyl-4-nitrocyclohexene (3d). Pale-yellow solid (dr = 52/48). Major isomer: 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 8.4 Hz, 2H), 7.00 (d, J = 8.4 Hz, 2H), 4.23 (dq, J = 7.2, 10.8 Hz, 1H), 4.20 (dq, J = 7.2, 10.8 Hz, 1H), 3.94 (d, J = 7.2 Hz,1H), 2.7–3.1 (m, 3H), 2.36 (br d, J = 18.0 Hz, 1H), 1.72 (s, 3H), 1.68 (s, 3H), 1.21 (dd, J = 7.2, 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 165.9 (C), 138.5 (C), 131.8 (CH), 130.3 (CH), 126.1 (C), 122.0 (C), 121.4 (C), 94.9 (C), 63.0 (CH2), 43.6 (CH), 37.3 (CH2), 34.1 (CH2), 19.4 (CH3), 18.4 (CH3), 13.8 (CH3). Minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.39 (d, J = 8.4 Hz, 2H), 7.04 (d, J = 8.4 Hz, 2H), 4.08 (q, J = 7.2 Hz, 2H), 4.03 (br d, J = 7.6 Hz, 1H), 2.7–3.1 (m, 3H), 2.26 (br d, J = 19.2 Hz, 1H), 1.72 (s, 3H), 1.67 (s, 3H), 1.56 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 165.6 (C) 139.1 (C), 131.9 (CH), 130.3 (CH), 125.5 (C), 122.0 (C), 121.4 (C), 95.2 (C), 62.8 (CH2), 42.8 (CH), 35.9 (CH2), 34.1 (CH2), 19.2 (CH3), 18.5 (CH3), 13.8 (CH3). IR (KBr/cm−1) 1752, 1554; HRMS (ESI/TOF) calculated for (M + H+) C17H20BrNO4: 382.0649, found: 382.0644.
4-Ethoxycarbonyl-5-[4-(trifluoromethyl)phenyl]-1,2-dimethyl-4-nitrocyclohexene (3e). Pale-yellow oil (dr = 62/38). Major isomer: 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 8.4 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H), 4.15 (d, J = 7.2 Hz, 1H), 4.08 (q, J = 7.2 Hz, 2H), 2.6–3.1 (m, 3H), 2.31 (d, J = 18.4 Hz, 1H), 1.74 (s, 3H), 1.69 (s, 3H), 1.13 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 165.6 (C), 144.2 (C), 130.2 (C, q, J = 33 Hz), 129.1 (CH), 125.7 (CH, q, J = 4 Hz), 125.5 (C), 124.1 (C, q, J = 270 Hz), 121.6 (C), 95.1 (C), 62.9 (CH2), 43.2 (CH), 35.9 (CH2), 34.1 (CH2), 19.2 (CH3), 18.5 (CH3), 13.7 (CH3). Minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.32 (d, J = 7.6 Hz, 2H), 7.27 (d, J = 7.6 Hz, 2H), 4.25 (dq, J = 7.2, 10.8 Hz, 1H), 4.21 (dq, J = 7.2, 10.8 Hz, 1H), 4.04 (dd, J = 2.8, 7.2 Hz, 1H), 2.6–3.1 (m, 3H), 2.41 (d, J = 18.8 Hz, 1H), 1.74 (s, 3H), 1.69 (s, 3H), 1.21 (dd, J = 7.2, 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 165.8 (C), 144.2 (C), 130.2 (C, q, J = 32 Hz), 129.1 (CH), 126.1 (C), 125.6 (CH, q, J = 4 Hz), 124.1 (C, q, J = 271 Hz), 121.5 (C), 94.8 (C), 63.1 (CH2), 44.0 (CH), 37.2 (CH2), 33.9 (CH2), 19.4 (CH3), 18.4 (CH3), 13.8 (CH3); IR (KBr/cm−1) 1753, 1556, 1167, 1326; HRMS (ESI/TOF) calculated for (M + Na+) C18H19NO4F3Na: 394.1237, found: 339.1237.
4-Ethoxycarbonyl-1,2-dimethyl-4-nitro-5-[4-nitrophenyl]cyclohexene (3f). Pale-yellow oil (dr = 65/35). Major isomer: 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 8.7 Hz, 2H), 7.37 (d, J = 8.7 Hz, 2H), 4.18 (d, J = 7.3 Hz, 1H), 4.10 (q, J = 7.3 Hz, 2H), 2.80–3.09 (m, 3H), 2.33 (d, J = 17.8 Hz, 1H), 1.75 (s, 3H), 1.70 (s, 3H), 1.22 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 165.3 (C), 147.6 (C), 147.4 (C), 129.7 (CH), 125.4 (C), 124.0 (CH), 121.7 (C), 94.8 (C), 63.1 (CH2), 43.3 (CH), 35.8 (CH2), 34.3 (CH2), 19.2 (CH3), 18.6 (CH3), 13.9 (CH3). Minor isomer: 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 8.7 Hz, 2H), 7.32 (d, J = 8.7 Hz, 2H), 4.25 (dq, J = 7.3, 10.5 Hz, 1H), 4.22 (dq, J = 7.3, 10.5 Hz, 1H), 2.80–3.09 (m, 3H), 2.40 (d, J = 18.3 Hz, 1H), 1.76 (s, 3H), 1.71 (s, 3H), 1.22 (dd, J = 7.3, 7.3 Hz, 3H), One signal could not be observed presumably due to overlapping; 13C NMR (101 MHz, CDCl3) δ 165.6 (C), 147.6 (C), 146.8 (C), 129.7 (CH), 125.9 (C), 123.8 (CH), 121.6 (C), 94.6 (C), 63.3 (CH2), 43.9 (CH), 37.0 (CH2), 34.2 (CH2), 19.4 (CH3), 18.4 (CH3), 13.9 (CH3); HRMS (ESI/TOF) calculated for (M + Na+) C17H20N2O6Na: 371.1214, found: 371.1210.
4-Ethanoyl-4-ethoxycarbonyl-5-(4-methylphenyl)-1,2-dimethylcyclohexene (6). Pale-yellow oil (dr = 58/42). Major isomer: 1H NMR (400 MHz, CDCl3) δ 7.04–6.99 (m, 4H), 4.01 (dq, J = 7.3, 10.6 Hz, 1H), 4.06 (dq, J = 7.3, 10.6 Hz, 1H), 3.77 (br d, J = 7.3 Hz, 1H), 2.87–2.43 (m, 3H), 2.29 (s, 3H), 2.17–2.10 (m, 1H), 2.08 (s, 3H), 1.74 (s, 3H), 1.64 (s, 3H), 1.18 (dd, 7.3, 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 204.0 (C), 170.5 (C), 140.0 (C), 136.4 (C), 129.0 (CH), 128.5 (CH), 126.7 (C), 122.5 (C), 64.1 (C), 61.3 (CH2), 41.7 (CH), 36.4 (CH2), 32.4 (CH2), 26.5 (CH3), 21.1 (CH3), 19.6 (CH3), 18.7 (CH3), 14.1 (CH3). Minor isomer: 1H NMR (400 MHz, CDCl3) δ 7.04–6.99 (m, 4H), 4.18 (dq, J = 7.3, 10.6 Hz, 1H), 4.14 (dq, J = 7.3, 10.6 Hz, 1H), 3.77 (br d, J = 7.3 Hz, 1H), 2.87–2.43 (m, 3H), 2.28 (s, 3H), 2.17–2.10 (m, 1H), 1.95 (s, 3H), 1.74 (s, 3H), 1.65 (s, 3H), 1.19 (dd, 7.3, 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 205.4 (C), 171.7 (C), 139.3 (C), 136.5 (C), 129.1 (CH), 128.4 (CH), 125.6 (C), 123.3 (C), 64.2 (C), 61.4 (CH2), 42.7 (CH), 37.0 (CH2), 32.4 (CH2), 27.4 (CH3), 21.1 (CH3), 19.5 (CH3), 18.7 (CH3), 14.1 (CH3); HRMS (ESI/TOF) calculated for (M + Na+) C20H26O3Na: 337.1774, found: 337.1774.
4-Cyano-4-methoxycarbonyl-1,2-dimethyl-5-(4-methylphenyl)cyclohexene (7). Pale-yellow oil (dr = 100/0). 1H NMR (400 MHz, CDCl3) δ 7.25 (d, J = 7.6 Hz, 2H), 7.12 (d, J = 7.6 Hz, 2H), 3.49 (s, 3H), 3.22 (dd, J = 7.2, 12.0 Hz, 1H), 2.92 (br d, J = 16.8 Hz, 1H), 2.73 (br dd, J = 17.6, 12.0 Hz, 1H), 2.46 (d, J = 16.8 Hz, 1H), 2.32 (s, 3H), 2.23 (dd, J = 7.2, 17.6 Hz, 1H), 1.71 (br s, 6H); 13C NMR (101 MHz, CDCl3) δ 169.4 (C), 137.7 (C), 136.1 (C), 129.4 (CH), 127.9 (CH), 126.5 (C), 121.0 (C), 118.7 (C), 53.2 (CH3), 50.6 (C), 45.9 (CH), 41.0 (CH2), 36.3 (CH2), 21.2 (CH3), 18.9 (CH3), 18.6 (CH3); IR (KBr/cm−1) 1740; HRMS (ESI/TOF) calculated for (M + H+) C18H22NO2: 284.1645, found: 284.1645.
5-Ethoxycarbonyl-6-(4-methylphenyl)-5-nitrobicyclo [2.2.1]hept-2-ene (9). Yellow oil (dr = 59/25/15/1). 1H NMR (400 MHz, CDCl3) Major isomer: δ 7.20 (d, J = 8.2 Hz, 2H), 7.09 (d, J = 8.2 Hz, 2H), 6.66 (dd, J = 3.2, 5.5 Hz, 1H), 6.07 (dd, J = 2.7, 5.5 Hz, 1H), 4.04 (d, J = 2.7 Hz, 1H), 3.76 (d, J = 2.7 Hz, 1H), 3.73 (dq, J = 7.3, 10.5 Hz, 1H), 3.61 (dq, J = 7.3, 10.5 Hz, 1H), 3.16 (br s, 1H), 2.72 (d, J = 9.6 Hz, 1H), 2.30 (s, 3H), 1.95 (d, J = 9.6 Hz, 1H), 0.82 (dd, J = 7.3, 7.3 Hz, 3H); Minor isomer 1: δ 7.02 (d, J = 8.2 Hz, 2H), 6.98 (d, J = 8.2 Hz, 2H), 6.66–6.69 (m, 1H), 6.49 (dd, J = 3.2, 5.5 Hz, 1H), 4.58 (d, J = 3.2 Hz, 1H), 4.30 (q, J = 6.9 Hz, 2H), 3.62–3.64 (m, 1H), 3.10–3.15 (m, 1H), 2.27 (s, 3H), 1.62–1.70 (m, 2H), 1.28 (t, J = 6.9 Hz, 3H); Minor isomer 2: δ 7.02–7.07 (m, 4H), 6.62–6.65 (m, 1H), 6.47 (dd, J = 2.7, 5.5 Hz, 1H), 4.74 (d, J = 3.2 Hz, 1H), 3.80–3.83 (m, 1H), 3.58–3.79 (m, 2H), 3.11–3.14 (m, 1H), 2.28 (s, 3H), 1.62–1.70 (m, 2H), 0.69 (t, J = 7.3 Hz, 3H); HRMS (ESI/TOF) calculated for (M + H+) C17H19NO4: 324.1206, found: 324.1196.
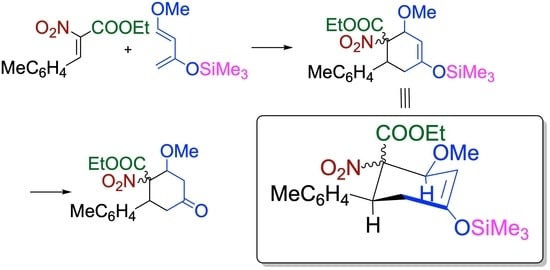
4-Ethoxycarbonyl-3-methoxy-5-(4-methylphenyl)-1-(trimethylsiloxy)-4-nitrocyclohexene (11). Pale-yellow oil (dr = 86/14). 1H NMR (400 MHz, CDCl3) δ 7.14 (d, J = 8.0 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 5.17 (d, J = 4.0 Hz, 1H), 4.54 (d, J = 4.0 Hz, 1H), 4.24 (dq, J = 7.2, 10.8 Hz, 1H), 4.16 (dq, J = 7.2, 10.8 Hz, 1H), 3.94 (dd, J = 6.4, 8.0 Hz, 1H), 3.41 (s, 3H), 2.38 (dd, J = 6.4, 18.0 Hz, 1H), 2.39 (dd, J = 8.0, 18.0 Hz, 1H), 2.32 (s, 3H), 1.23 (dd, J = 7.2, 7.2 Hz, 3H), 0.26 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 164.5 (C), 153.5 (C), 137.7 (C), 135.3 (C), 129.4 (CH), 128.9 (CH), 100.3 (CH), 96.2 (C), 76.4 (CH), 62.3 (CH2), 57.4 (CH3), 42.7 (CH), 35.3 (CH2), 21.2 (CH3), 14.0 (CH3), 0.4 (CH3); IR (KBr/cm−1) 1766, 1733, 1549, 1244, 1220, 1084, 849; HRMS (ESI/TOF) calculated for (M + Na+) C20H29NO6SiNa: 430.1656, found: 430.1651.
4-Ethoxycarbonyl-3-methoxy-5-(4-methylphenyl)-4-nitrocyclohexanone (12). Pale-yellow oil (dr = 95/5). 1H NMR (400 MHz, CD3Cl) δ 7.06 (s, 4H), 4.49 (dd, J = 4.1, 4.1 Hz, 1H), 4.27 (dq, J = 7.3, 11.0 Hz, 1H), 4.21 (dd, J = 5.5, 11.4 Hz, 1H), 4.20 (dq, J = 7.3, 11.0 Hz, 1H), 3.39 (dd, J = 4.1, 15.6 Hz, 1H), 3.35 (3H, s), 2.90 (dd, J = 11.4, 15.6 Hz, 1H), 2.81 (ddd, J = 1.8, 4.1, 15.6 Hz, 1H), 2.72 (ddd, J = 1.8, 5.5, 15.6 Hz, 1H), 2.28 (s, 3H), 1.23 (dd, J = 7.3, 7.3 Hz, 3H); 13C NMR (101 MHz) δ 205.5 (C), 163.9 (C), 137.9 (C), 134.0 (C), 128.9 (CH), 128.6 (CH), 95.5 (C), 79.4 (CH), 62.6 (CH2), 57.6 (CH), 43.9 (CH3), 43.5 (CH2), 41.1 (CH2), 20.9 (CH3), 13.7 (CH3); IR (KBr/cm−1) 1766, 1729, 1548, 1243, 1083; HRMS (ESI/TOF) calculated for (M + H+) C17H22NO6: 336.1442, found: 336.1443.
3-Ethoxycarbonyl-4-(4-methylphenyl)-3-nitro-6-[(2,4dinitrophenyl)hydrazino)]cyclohexene (13). Orange needles, m.p. 110–111 °C. 1H NMR (400 MHz, CD3Cl) δ 11.24 (br s, 1H), 9.11 (d, J = 2.4 Hz, 1H), 8.37 (dd, J = 2.4, 9.6 Hz, 1H), 8.06 (d, J = 9.6 Hz, 1H), 7.05 (s, 4H), 6.83 (d, J = 9.2 Hz, 1H), 6.65 (dd, J = 0.9, 9.2 Hz, 1H), 4.42 (dq, J = 7.3, 10.1 Hz, 1H), 4.42 (ddd, J = 0.9, 2.7, 6.9 Hz, 1H), 4.30 (dq, J = 7.3, 10.1 Hz, 1H), 3.42 (dd, J = 6.9, 17.4 Hz, 1H), 3.04 (dd, J = 2.7, 17.4 Hz, 1H), 2.27 (s, 3H), 1.29 (dd, J = 7.3, 7.3 Hz, 3H); 13C NMR (101 MHz, CD3Cl) δ 164.8 (C), 148.1 (C), 144.4 (C), 139.2(C), 138.7 (C), 134.4 (C), 132.1 (CH), 130.5 (C), 130.3 (CH), 129.8 (CH), 128.3 (CH), 127.1 (CH), 123.4 (CH), 116.8 (CH), 93.8 (C), 63.9 (CH2), 43.3 (CH), 28.9 (CH2), 21.2 (CH3), 13.9 (CH3); IR (KBr/cm−1) 1750, 1616, 1337, 771; HRMS (ESI/TOF) calculated for (M + H+) C22H22NO2: 484.1463, found: 484.1453.