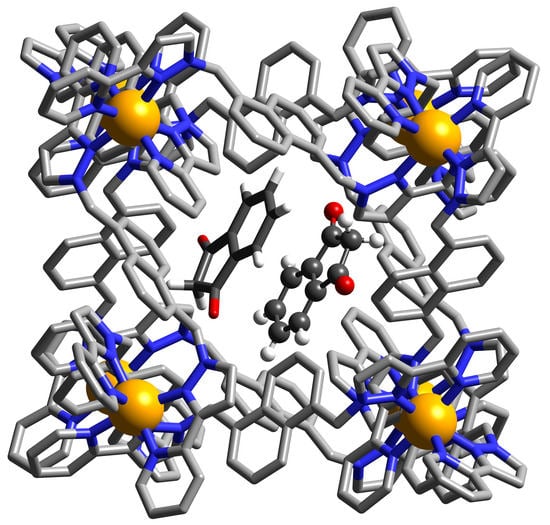
Catalysis of an Aldol Condensation Using a Coordination Cage
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, C.J.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular catalysis in metal-ligand cluster hosts. Chem. Rev. 2015, 115, 3012–3025. [Google Scholar] [CrossRef]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Klosterman, J.K.; Fujita, M. Functional molecular flasks: New properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. 2009, 48, 3418–3438. [Google Scholar] [CrossRef] [PubMed]
- Otte, M. Size-selective molecular flasks. ACS Catal. 2016, 6, 6491–6510. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Nitschke, J.R. Functional capsules via subcomponent self-assembly. Acc. Chem. Res. 2018, 51, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; He, C.; Zhao, L.; Duan, C. Photochemical properties of host-guest supramolecular systems with structurally confined metal-organic capsules. Acc. Chem. Res. 2019, 52, 100–109. [Google Scholar] [CrossRef]
- Hong, C.M.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Self-Assembled Tetrahedral Hosts as Supramolecular Catalysts. Acc. Chem. Res. 2018, 51, 2447–2455. [Google Scholar] [CrossRef]
- Catti, L.; Zhang, Q.; Tiefenbacher, K. Advantages of catalysis in self-assembled molecular capsules. Chem. Eur. J. 2016, 22, 9060–9066. [Google Scholar] [CrossRef]
- Gao, W.-X.; Zhang, H.-N.; Jin, G.-X. Supramolecular catalysis based on discrete heterometallic coordination-driven metallacycles and metallacages. Coord. Chem. Rev. 2019, 386, 69–84. [Google Scholar] [CrossRef]
- Ward, M.D.; Hunter, C.A.; Williams, N.H. Coordination cages based on bis(pyrazolylpyridine) ligands: Structures, dynamic behavior, guest binding, and catalysis. Acc. Chem. Res. 2018, 51, 2073–2082. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, M.; Tamura, M.; Fujita, M. Diels-alder in aqueous molecular hosts: Unusual regioselectivity and efficient catalysis. Science 2006, 312, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Horiuchi, S.; Fujita, M. Naphthalene Diels-Alder in a self-assembled molecular flask. J. Am. Chem. Soc. 2010, 132, 2866–2867. [Google Scholar] [CrossRef]
- Kang, J.; Rebek, J., Jr. Acceleration of a Diels–Alder reaction by a self-assembled molecular capsule. Nature 1997, 385, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hilmersson, G.; Santamaría, J.; Rebek, J., Jr. Diels-Alder reactions through reversible encapsulation. J. Am. Chem. Soc. 1998, 120, 3650–3656. [Google Scholar] [CrossRef]
- Kaphan, D.M.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Enabling new modes of reactivity via constrictive binding in a supramolecular-assembly-catalyzed aza-Prins cyclization. J. Am. Chem. Soc. 2015, 137, 9202–9205. [Google Scholar] [CrossRef] [PubMed]
- Hart-Cooper, W.M.; Zhao, C.; Triano, R.M.; Yaghoubi, P.; Ozores, H.L.; Burford, K.N.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. The effect of host structure on the selectivity and mechanism of supramolecular catalysis of Prins cyclizations. Chem. Sci. 2015, 6, 1383–1393. [Google Scholar] [CrossRef] [Green Version]
- Hastings, C.J.; Bergman, R.G.; Raymond, K.N. Origins of large rate enhancements in the Nazarov cyclisation catalysed by supramolecular encapsulation. Chem. Eur. J. 2014, 20, 3966–3973. [Google Scholar] [CrossRef]
- Hastings, C.J.; Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Enzymelike catalysis of the Nazarov cyclization by supramolecular encapsulation. J. Am. Chem. Soc. 2010, 132, 6938–6940. [Google Scholar] [CrossRef] [Green Version]
- Fiedler, D.; van Halbeek, H.; Bergman, R.G.; Raymond, K.N. Supramolecular catalysis of unimolecular rearrangements: Substrate scope and mechanistic insights. J. Am. Chem. Soc. 2006, 128, 10240–10252. [Google Scholar] [CrossRef]
- Cullen, W.; Takezawa, H.; Fujita, M. Demethylenation of cyclopropanes via photoinduced guest-to-host electron transfer in an M6L4 cage. Angew. Chem. Int. Ed. 2019, 58, 9171. [Google Scholar] [CrossRef]
- Dalton, D.M.; Ellis, S.R.; Nichols, E.M.; Mathies, R.A.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular [Ga4L6]12− cage photosensitizes 1,3-rearrangement of encapsulated guest via photoinduced electron transfer. J. Am. Chem. Soc. 2015, 137, 10128–10131. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Takezawa, H.; Fujita, M. Photo-driven anti-Markovnikoz alkyne hydration in self-assembled hollow complexes. Chem. Commun. 2011, 47, 10960–10962. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Nishijima, Y.; Fujita, M. Unusual photoreaction of triquinacene within self-assembled hosts. Chem. Asian J. 2012, 7, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Martí-Centelles, V.; Lawrence, A.L.; Lusby, P.J. High activity and efficient turnover by a simple, self-assembled “artificial Diels–Alderase”. J. Am. Chem. Soc. 2018, 140, 2862–2868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.-X.; Li, S.-C.; Yan, D.-N.; Zhou, L.-P.; Guo, F.; Sun, Q.-F. Water-soluble redox-active cage hosting polyoxometalates for selective desulfurization catalysis. J. Am. Chem. Soc. 2018, 140, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Bender, T.A.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. A supramolecular strategy for selective catalytic hydrogenation independent of remote chain length. J. Am. Chem. Soc. 2019, 141, 11806–11810. [Google Scholar] [CrossRef]
- Jongkind, L.J.; Caumes, X.; Hartendorp, A.P.T.; Reek, J.N.H. Ligand template strategies for catalyst encapsulation. Acc. Chem. Res. 2018, 51, 2115–2128. [Google Scholar] [CrossRef]
- Tidmarsh, I.S.; Faust, T.B.; Adams, H.; Harding, L.P.; Russo, L.; Clegg, W.; Ward, M.D. Octanuclear cubic coordination cages. J. Am. Chem. Soc. 2008, 130, 15167–15175. [Google Scholar] [CrossRef]
- Cullen, W.; Misuraca, M.C.; Hunter, C.A.; Williams, N.H.; Ward, M.D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 2016, 8, 231–236. [Google Scholar] [CrossRef]
- Cullen, W.; Metherell, A.J.; Wragg, A.B.; Taylor, C.G.P.; Williams, N.H.; Ward, M.D. Catalysis in a cationic coordination cage using a cavity-bound guest and surface-bound anions: Inhibition, activation, and autocatalysis. J. Am. Chem. Soc. 2018, 140, 2821–2828. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, M.; Turega, S.; Stephenson, A.; Hunter, C.A.; Ward, M.D. Quantification of solvent effects on molecular recognition in polyhedral coordination cage hosts. Chem. Sci. 2013, 4, 2744–2751. [Google Scholar] [CrossRef] [Green Version]
- Turega, S.; Cullen, W.; Whitehead, M.; Hunter, C.A.; Ward, M.D. Mapping the internal recognition surface of an octanuclear coordination cage using guest libraries. J. Am. Chem. Soc. 2014, 136, 8475–8483. [Google Scholar] [CrossRef] [PubMed]
- Metherell, A.J.; Cullen, W.; Williams, N.H.; Ward, M.D. Binding of hydrophobic guests in a coordination cage cavity is driven by liberation of ‘high-energy’ water. Chem. Eur. J. 2018, 24, 1554–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Hahn, B.P.; Jones, R.A.; Wong, W.-K.; Stevenson, K.J. Synthesis of an octanuclear Eu(III) cage from Eu42+: Chloride anion encapsulation, luminescence, and reversible MeOH adsorption via a porous supramolecular architecture. Inorg. Chem. 2007, 46, 7050–7054. [Google Scholar] [CrossRef] [PubMed]
- Bilyachenko, A.N.; Khrustalev, V.N.; Zubavichus, Y.V.; Vologzhanina, A.V.; Astakhov, G.S.; Gutsul, E.I.; Shubina, E.S.; Levitsky, M.M. High-nuclearity (Cu8-based) cage silsesquioxanes: Synthesis and structural study. Cryst Growth Des. 2018, 18, 2452–2457. [Google Scholar] [CrossRef]
- Elahi, S.M.; Rajasekharan, M.V. Spherical octanuclear clusters in a Na-Ln-dipic system: Encapsulation of a nitrate ion and incorporation of water nonamers and dodecamers. CrystEngComm 2015, 17, 7191–7198. [Google Scholar] [CrossRef]
- Freudenreich, J.; Dalvit, C.; Süss-Fink, G.; Therrien, B. Encapsulation of photosensitizers in hexa- and octanuclear organometallic cages: Synthesis and characterization of carceplex and host-guest systems in solution. Organometallics 2013, 32, 3018–3033. [Google Scholar] [CrossRef]
- Cullen, W.; Turega, S.; Hunter, C.A.; Ward, M.D. Virtual screening for high affinity guests for synthetic supramolecular receptors. Chem. Sci. 2015, 6, 2790–2794. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.G.P.; Cullen, W.; Collier, O.M.; Ward, M.D. A quantitative study of the effects of guest flexibility on binding inside a coordination cage host. Chem. Eur. J. 2017, 23, 206–213. [Google Scholar] [CrossRef]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Acid catalysis in basic solution: A supramolecular host promotes orthoformate hydrolysis. Science 2007, 316, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. The acid hydrolysis mechanism of acetals catalyzed by a supramolecular assembly in basic solution. J. Org. Chem. 2009, 74, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Making amines strong bases: Thermodynamic stabilization of protonated guests in a highly-charged supramolecular host. J. Am. Chem. Soc. 2007, 129, 11459–11467. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Sigalov, M.; Becker, J.Y.; Ellern, A.; Khodorkovsky, V. Self-condensation of 1,3-indandione: A reinvestigation. Eur. J. Org. Chem. 2000, 2047–2055. [Google Scholar] [CrossRef]
- Sigalov, M.; Krief, P.; Shapiro, L.; Khodorkovsky, V. Inter- and intramolecular C-H···O bonding in the anions of 1,3-indandione derivatives. Eur. J. Org. Chem. 2008, 673–683. [Google Scholar] [CrossRef]
- Wislicenus, W. Ueber die vereinigung verschiedener ester durch natrium. Ber. Dtsch. Chem. Ges. 1887, 20, 589. [Google Scholar] [CrossRef] [Green Version]
- Wislicenus, W. Einwirkung von essigester auf phtalsäureester. Annalen 1888, 246, 347–355. [Google Scholar]
- Allan, D.R.; Nowell, H.; Barnett, S.A.; Warren, M.R.; Wilcox, A.; Christensen, J.; Saunders, L.K.; Peach, A.; Hooper, M.T.; Zaja, L.; et al. A novel dial air-bearing fixed-χ diffractometer for small-molecular single-crystal X-ray diffraction on beamline I-19 at Diamond Light Source. Crystals 2017, 7, 336. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.G.P.; Argent, S.P.; Ludden, M.D.; Piper, J.R.; Mozaceanu, C.; Barnett, S.A.; Ward, M.D. One guest or two? A crystallographic and solution study of guest binding in a cubic coordination cage. Chem. Eur. J. 2019, in press. [Google Scholar] [CrossRef]
- Turega, S.; Whitehead, M.; Hall, B.R.; Meijer, A.J.H.M.; Hunter, C.A.; Ward, M.D. Shape-, size- and functional group-selective binding of small organic guests in a paramagnetic coordination cage. Inorg. Chem. 2013, 52, 1122–1132. [Google Scholar] [CrossRef]
- Mecozzi, S.; Rebek, J. The 55% solution: A formula for molecular recognition in the liquid state. Chem. Eur. J. 1998, 4, 1016–1022. [Google Scholar] [CrossRef]
- Rebek, J. Molecular behaviour in small spaces. Acc. Chem. Res. 2009, 42, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Puttreddy, R.; Beyeh, N.K.; Kalenius, E.; Ras, R.H.A.; Rissanen, K. 2-Methylresorcinarene: A very high packing coefficient in a mono-anion based dimeric capsule and the X-ray crystal structure of the tetra-anion. Chem. Commun. 2016, 52, 8115–8118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yang, D.; Zhao, J.; Hou, L.; Sessler, J.L.; Yang, X.-J.; Wu, B. Controlling the recognition and reactivity of alkyl ammonium guests using an anion coordination-based tetrahedral cage. J. Am. Chem. Soc. 2018, 140, 5248–5256. [Google Scholar] [CrossRef] [PubMed]
- Djemili, R.; Kocher, L.; Durot, S.; Peuronen, A.; Rissanen, K.; Heitz, V. Positive allosteric control of guests encapsulation by metal binding to covalent porphyrin cages. Chem. Eur. J. 2019, 25, 1481–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metherell, A.J.; Ward, M.D. Geometric isomerism in coordination cages based on tris-chelate vertices: A tool to control both assembly and host/guest chemistry. Dalton Trans. 2016, 45, 16096–16111. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C. Sruct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.G.P.; Metherell, A.J.; Argent, S.P.; Ashour, F.M.; Williams, N.H.; Ward, M.D. Coordination cage catalysed hydrolysis of organophosphates: Cavity or surface based? Chem. Eur. J. 2019, in press. [Google Scholar] [CrossRef]
- Das, P.; Kumar, A.; Howlader, P.; Mukherjee, P.S. A self-assembled trigonal prismatic molecular vessel for catalytic dehydration reactions in water. Chem. Eur. J. 2017, 23, 12565–12574. [Google Scholar] [CrossRef]







| Empirical formula | C398.15H435.65B16Co8F64N74.85O39.35 |
| Formula weight | 8759.54 |
| T/K | 100(1) |
| Crystal system | Monoclinic |
| Space group | C2/c |
| Crystal size/mm3 | 0.04 × 0.04 × 0.04 |
| a/Å | 32.77108(17) |
| b/Å | 30.00687(16) |
| c/Å | 40.3365(2) |
| β/degrees | 96.1279(5) |
| V/Å3 | 39,438.6(3) |
| Z | 4 |
| ρcalc/g cm−3 | 1.475 |
| μ/mm−1 | 0.405 |
| Radiation | Synchrotron (λ = 0.6889) |
| Reflections collected | 337,794 |
| Data/restraints/parameters | 62,798/5962/2330 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0651, wR2 = 0.2094 |
| Final R indexes (all data) | R1 = 0.1117, wR2 = 0.2292 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozaceanu, C.; Taylor, C.G.P.; Piper, J.R.; Argent, S.P.; Ward, M.D. Catalysis of an Aldol Condensation Using a Coordination Cage. Chemistry 2020, 2, 22-32. https://doi.org/10.3390/chemistry2010004
Mozaceanu C, Taylor CGP, Piper JR, Argent SP, Ward MD. Catalysis of an Aldol Condensation Using a Coordination Cage. Chemistry. 2020; 2(1):22-32. https://doi.org/10.3390/chemistry2010004
Chicago/Turabian StyleMozaceanu, Cristina, Christopher G. P. Taylor, Jerico R. Piper, Stephen P. Argent, and Michael D. Ward. 2020. "Catalysis of an Aldol Condensation Using a Coordination Cage" Chemistry 2, no. 1: 22-32. https://doi.org/10.3390/chemistry2010004
APA StyleMozaceanu, C., Taylor, C. G. P., Piper, J. R., Argent, S. P., & Ward, M. D. (2020). Catalysis of an Aldol Condensation Using a Coordination Cage. Chemistry, 2(1), 22-32. https://doi.org/10.3390/chemistry2010004