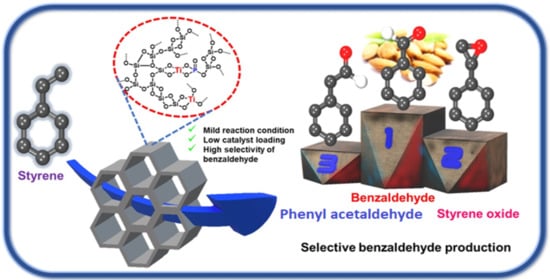
Selective Styrene Oxidation Catalyzed by Phosphate Modified Mesoporous Titanium Silicate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Synthesis of STP-1
2.4. Styrene Oxidation Catalysis
2.5. Catalyst Activation
3. Results
4. Discussion
4.1. Catalysis
4.2. Plausible Reaction Mechanism
4.3. Recyclability Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trost, B.M. Comprehensive Organic Synthesis; Pergamon: New York, NY, USA, 1991. [Google Scholar]
- Corma, A.; Serra, J.M.; Serna, P.; Valero, S.; Argente, E.; Botti, V. Optimisation of olefin epoxidation catalysts with the application of high-throughput and genetic algorithms assisted by artificial neural networks (softcomputing techniques). J. Catal. 2005, 229, 513–524. [Google Scholar] [CrossRef]
- Parker, R.E.; Isaacs, N.S. Mechanisms of Epoxide Reactions. Chem. Rev. 1959, 59, 737–799. [Google Scholar] [CrossRef]
- Corma, A.; Navarro, M.T.; Pariente, J.P. Synthesis of an Ultralarge Pore Titanium Silicate Isomorphous to MCM-41 and Its Application as a Catalyst for Selective Oxidation of Hydrocarbons. J. Chem. Soc. Chem. Commun. 1994, 2, 147–148. [Google Scholar] [CrossRef]
- Bhaumik, A.; Kumar, R. Titanium silicate molecular sieve (TS-1)/H2O2 induced triphase catalysis in the oxidation of hydrophobic organic compounds with significant enhancement of activity and para selectivity. J. Chem. Soc. Chem. Commun. 1995, 3, 249–250. [Google Scholar] [CrossRef]
- Kholdeeva, O.; Maksimchuk, N. Metal-Organic Frameworks in Oxidation Catalysis with Hydrogen Peroxide. Catalysts 2021, 11, 283. [Google Scholar] [CrossRef]
- Bailey, P.S.; Hwang, H.H.; Chiang, C.Y. Mechanisms of Epoxidation during Ozonation of Carbon-Carbon Double Bonds. J. Org. Chem. 1985, 50, 231–234. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, Z. Catalyst-Free Selective Oxidation of Diverse Olefins to Carbonyls in High Yield Enabled by Light under Mild Conditions. Org. Lett. 2019, 21, 7726–7730. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Dubois, G.; Stack, T.D.P. Efficient Epoxidation of Electron-Deficient Olefins with a Cationic Manganese Complex. J. Am. Chem. Soc. 2003, 125, 5250–5251. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef]
- Bhaumik, A.; Mukherjee, P.; Kumar, R. Triphase catalysis over titanium-silicate molecular sieves under solvent-free conditions—I. Direct hydroxylation of benzene. J. Catal. 1998, 178, 101–107. [Google Scholar] [CrossRef]
- Drozd, V.A.; Ottenbacher, R.V.; Bryliakov, K.P. Asymmetric Epoxidation of Olefins with Sodium Percarbonate Catalyzed by Bis-Amino-Bis-Pyridine Manganese Complexes. Molecules 2022, 27, 2538. [Google Scholar] [CrossRef]
- Sun, C.; Liu, H. Highly Selective Oxidation of styrene to styrene Oxide over a Tetraphenylporphyrin-Bridged Silsesquioxane-Based Hybrid Porous Polymer. ACS Appl. Mater. Interfaces 2022, 4, 5471–5481. [Google Scholar] [CrossRef]
- Liu, R.; Qu, J. Review on heterogeneous oxidation and adsorption for arsenic removal from drinking water. J. Environ. Sci. 2021, 110, 178–188. [Google Scholar] [CrossRef]
- Jørgensen, K.A. Transition-Metal-Catalyzed Epoxidations. Chem. Rev. 1989, 89, 431–458. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Van Doorn, J.A. Metal-Catalyzed Epoxidation of Olefins with Organic Hydroperoxides. I. A Comparison of Various Metal Catalysts. J. Catal. 1973, 31, 427–437. [Google Scholar] [CrossRef]
- Xu, D.; Sun, Q.; Lin, J.; Sun, W. Ligand Regulation for Manganese-Catalyzed Enantioselective Epoxidation of Olefins without Acid. Chem. Commun. 2020, 56, 13101–13104. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.E. Oxidation of Olefins in the Presence of Transition Metal Complexes. Adv. Chem. Ser. 1974, 132, 64–89. [Google Scholar]
- Oloo, W.N.; Banerjee, R.; Lipscomb, J.D.; Que, L. Equilibrating (L)FeIII–OOAc and (L)FeV(O) Species in Hydrocarbon Oxidations by Bio-Inspired Nonheme Iron Catalysts Using H2O2 and AcOH. J. Am. Chem. Soc. 2017, 139, 17313–17326. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Huber, G.W. Catalysis Science & Technology Ethylene Oligomerization into Linear Olefins. Catal. Sci. Technol. 2022, 12, 3639–3649. [Google Scholar]
- Xiong, C.; Liang, Y.; Zhou, X.; Xue, C.; Ji, H. Facile Synthesis of a Mo-Based TiO2 Catalyst via a Redox Strategy for High Value-Added Conversion of Olefin. Fuel 2023, 332, 126172. [Google Scholar] [CrossRef]
- Jibowu, T. A Review on Nanoporous Metals. Front. Nanosci. Nanotechnol. 2016, 2, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.M.; Zhang, X.J. Enhancing the Stability of Metal-Organic Framework via Ligand Modification: Scalable Synthesis and High Selectivity of CO2 Sorption Property. CrystEngComm 2022, 25, 467–472. [Google Scholar] [CrossRef]
- Song, B.Y.; Zhang, X.F.; Huang, J.; Cheng, X.L.; Deng, Z.P.; Xu, Y.M.; Huo, L.H.; Gao, S. Porous Cr2O3 Architecture Assembled by Nano-Sized Cylinders/Ellipsoids for Enhanced Sensing to Trace H2S Gas. ACS Appl. Mater. Interfaces 2022, 14, 22302–22312. [Google Scholar] [CrossRef] [PubMed]
- Sodha, V.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Bandyopadhyay, R.; Sridewi, N. Comprehensive Review on Zeolite-Based Nanocomposites for Treatment of Effluents from Wastewater. Nanomaterials 2022, 12, 3199. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.X.; Wei, Z.W.; Chen, C.X.; Xiong, X.H.; Xiong, Y.Y.; Zeng, Z.; Wang, W.; Jiang, J.J.; Fan, Y.N.; Su, C.Y. High Water Adsorption MOFs with Optimized Pore-Nanospaces for Autonomous Indoor Humidity Control and Pollutants Removal. Angew. Chem. Int. Ed. 2022, 61, e202112097. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beckt, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid- Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa, M.; Sierra, L.; Patarin, J.; Guth, J. Morphology and Porosity Characteristics Control of SBA-16 Mesoporous Silica. Effect of the Triblock Surfactant Pluronic F127 Degradation during the Synthesis. Solid State Sci. 2005, 7, 990–997. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.K.; Ryoo, R. Synthesis of MCM-48 Single Crystals. Chem. Commun. 1998, 1, 259–260. [Google Scholar] [CrossRef]
- Bonino, F.; Damin, A.; Ricchiardi, G.; Ricci, M.; Spano, G.; D’Aloisio, R.; Zecchina, A.; Lamberti, C.; Prestipino, C.; Bordiga, S. Ti-peroxo species in the TS-1/H2O2/H2O system. J. Phys. Chem. B 2004, 108, 3573–3583. [Google Scholar] [CrossRef]
- Gordon, C.P.; Engler, H.; Tragl, A.S.; Plodinec, M.; Lunkenbein, T.; Berkessel, A.; Teles, J.H.; Parvulescu, A.N.; Coperet, C. Efficient epoxidation over dinuclear sites in titanium silicalite-1. Nature 2020, 586, 708–710. [Google Scholar] [CrossRef]
- Zhang, M.; Ren, S.Y.; Guo, Q.X.; Shen, B.J. Synthesis of hierarchically porous zeolite TS-1 with small crystal size and its performance of 1-hexene epoxidation reaction. Microporous Mesoporous Mater. 2021, 326, 111395. [Google Scholar] [CrossRef]
- Bhaumik, A.; Inagaki, S. Mesoporous titanium phosphate molecular sieves with ion-exchange capacity. J. Am. Chem. Soc. 2001, 123, 691–696. [Google Scholar] [CrossRef]
- Alvaro, M.; Corma, A.; Das, D.; Fornes, V.; Garcia, H. “Nafion”—functionalized mesoporous MCM-41 silica shows high activity and selectivity for carboxylic acid esterification and Friedel-Crafts acylation reactions. J. Catal. 2005, 231, 48–55. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Bhaumik, A.; Tatsumi, T. Organically Modified Titanium-Rich Ti-MCM-41, Efficient Catalysts for Epoxidation Reactions. J. Catal. 2000, 189, 31–39. [Google Scholar] [CrossRef]
- Peng, R.; Zhao, D.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Room Temperature Synthesis of Ti-MCM-48 and Ti-MCM-41 Mesoporous Materials and Their Performance on Photocatalytic Splitting of Water. J. Phys. Chem. C 2012, 116, 1605–1613. [Google Scholar] [CrossRef]
- Rhee, C.H.; Lee, J.S. Preparation and Characterization of Titanium-Substituted MCM-41. Catal. Today 1997, 38, 213–219. [Google Scholar] [CrossRef]
- Schacht, P.; Noreña-Franco, L.; Ancheyta, J.; Ramírez, S.; Hernández-Pérez, I.; García, L.A. Characterization of Hydrothermally Treated MCM-41 and Ti-MCM-41 Molecular Sieves. Catal. Today 2004, 98, 115–121. [Google Scholar] [CrossRef]
- Yu, J.; Feng, Z.; Xu, L.; Li, M.; Xin, Q.; Liu, Z. Ti-MCM-41 Synthesized from Colloidal Silica and Titanium Trichloride: Synthesis, Characterization, and Catalysis. Chem. Mater. 2001, 13, 994–998. [Google Scholar] [CrossRef]
- Petersen, H.; Stegmann, N.; Fischer, M.; Zibrowius, B.; Radev, I.; Philippi, W.; Schmidt, W.; Weidenthaler, C. Crystal Structures of Two Titanium Phosphate-Based Proton Conductors: Ab Initio Structure Solution and Materials Properties. Inorg. Chem. 2022, 61, 2379–2390. [Google Scholar] [CrossRef]
- Li, C.; Xiong, G.; Xin, Q.; Liu, J.; Ying, P.; Feng, Z.; Li, J.; Yang, W.; Wang, Y.; Wang, G.; et al. UV Resonance Raman Spectroscopic Identification of Titanium Atoms in the Framework of TS-1 Zeolite. Angew. Chem. Int. Ed. 1999, 38, 2220–2222. [Google Scholar] [CrossRef]
- Boccuti, M.R.; Raol, K.M.; Zecchina, A.; Leofanti, G.; Petrini, G. Spectroscopic Characterization of Silicalite And Titanium-Silicalite. Struct. React. Surf. 1989, 48, 133–144. [Google Scholar]
- Hulea, V.; Dumitriu, E. Styrene Oxidation with H2O2 over Ti-Containing Molecular Sieves with MFI, BEA and MCM-41 Topologies. Appl. Catal. A Gen. 2004, 277, 99–106. [Google Scholar] [CrossRef]
- Das, S.; Chatterjee, S.; Mondal, S.; Modak, A.; Chandra, B.K.; Das, S.; Nessim, G.D.; Majee, A.; Bhaumik, A. Thiadiazole Containing N- and S-Rich Highly Ordered Periodic Mesoporous Organosilica for Efficient Removal of Hg(II) from Polluted Water. Chem. Commun. 2020, 56, 3963–3966. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chowdhury, B.; Bhaumik, A. Synthesis of Hollow Mesoporous Silica Nanospheroids with O/W Emulsion and Al(III) Incorporation and Its Catalytic. Catalysts 2023, 13, 354. [Google Scholar] [CrossRef]
- Sarkar, B.; Singha, R.K.; Tiwari, R.; Ghosh, S. Preparation of CeO2 Nanoparticles Supported on 1-D Silica Nanostructures for Room Temperature Selective Oxidation of Styrene. RSC Adv. 2014, 4, 5453–5456. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Cheng, J.; Li, Z.; Wang, H.; Sun, Q.; Han, B. Microporous and Mesoporous Materials Synthesis, Characterization and Catalytic Activity of Binary Metallic Titanium and Iron Containing Mesoporous Silica. Microporous Mesoporous Mater. 2012, 162, 51–59. [Google Scholar] [CrossRef]
- Sun, W.; Hu, J. Oxidation of Styrene to Benzaldehyde with Hydrogen Peroxide in the Presence of Catalysts Obtained by the Immobilization of H 3 PW 12 O 40 on SBA-15 Mesoporous Material. React. Kinet. Mech. Catal. 2016, 119, 305–318. [Google Scholar] [CrossRef]
- Gao, D.; Gao, Q. Selective Oxidation of Styrene to Benzaldehyde over VSB-5 and Isomorphously Substituted Cobalt VSB-5. Catal. Commun. 2007, 8, 681–685. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.; Jian, P.; Wang, L. Journal of Colloid and Interface Science Hierarchical Hollow Nickel Silicate Microflowers for Selective Oxidation of Styrene. J. Colloid Interface Sci. 2019, 553, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Pirouzmand, M.; Amini, M.M.; Safari, N. Immobilization of Iron Tetrasulfophthalocyanine on Functionalized MCM-48 and MCM-41 Mesoporous Silicas: Catalysts for Oxidation of Styrene. J. Colloid Interface Sci. 2008, 319, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Li, Y.; Liu, Q.; Xue, Z.; Wang, H.; Fan, Y.; Zhu, K. One-Step Construction of Hydrophobic MOFs @ COFs Core—Shell Composites for Heterogeneous Selective Catalysis. Adv. Sci. 2019, 1802365, 1802365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubis, S.; Yuliati, L.; Lee, S.L.; Sumpono, I.; Nur, H. Improvement of Catalytic Activity in Styrene Oxidation of Carbon-Coated Titania by Formation of Porous Carbon Layer. Chem. Eng. J. 2012, 209, 486–493. [Google Scholar] [CrossRef]
- Lignier, P.; Mangematin, S.; Morfin, F.; Rousset, J.L.; Caps, V. Solvent and Oxidant Effects on the Au/TiO2—Catalyzed Aerobic Epoxidation of Stilbene. Catal. Today 2008, 138, 50–54. [Google Scholar] [CrossRef]





| Entry | Catalyst | Catalyst Amount (wt%) | Temperature (°C) | Time (h) | Conversion (%) | SO Selectivity (%) | BA Selectivity (%) |
|---|---|---|---|---|---|---|---|
| 1 | Blank | - | 70 | 5 | 3.2 | 9.6 | 67.3 |
| 2 | STP-1 | 10 | 70 | 1 | 4.4 | 8.7 | 70.1 |
| 3 | STP-1 | 10 | 70 | 2 | 9.1 | 7.8 | 63.7 |
| 4 | STP-1 | 10 | 70 | 3 | 11.9 | 9.6 | 86.2 |
| 5 | STP-1 | 10 | 70 | 4 | 13.5 | 6.5 | 89.3 |
| 6 | STP-1 | 10 | 70 | 5 | 16.5 | 5.2 | 84.5 |
| 7 | STP-1 | 10 | 80 | 1 | 7.6 | 7.9 | 60.0 |
| 8 | STP-1 | 10 | 80 | 2 | 8.4 | 7.5 | 50.5 |
| 9 | STP-1 | 10 | 80 | 3 | 16.1 | 10.5 | 73.0 |
| 10 | STP-1 | 10 | 80 | 4 | 20.7 | 7.5 | 53.1 |
| 11 a | STP-1 | 10 | 80 | 10 | 25.2 | 6.3 | 47.2 |
| 12 a | STP-1 | 10 | 70 | 5 | 29.1 | 3.9 | 48.6 |
| 13 a | STP-1 | 10 | 70 | 10 | 32.8 | 4.6 | 93.8 |
| 14 b | STP-1 | 10 | 70 | 10 | 0.6 | n.d | 20.2 |
| 15 c | SP-1 | 10 | 70 | 10 | 6.6 | 22.5 | 71.6 |
| Entry | Catalyst | Reaction Condition | Styrene Conversion (%) | Benzaldehyde Selectivity (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Ti-MCM-41 (H2O2) | 70 °C, 5 h | 26.1 | 52.3 | [45] |
| 2 | Ti-Fe-MCM-41 (H2O2) | 70 °C, 12 h | 50 | 90.1 | [49] |
| 3 | TS-1 (H2O2) | 70 °C, 5 h | 21.1 | 26.5 | [45] |
| 4 | H3PW12O40/SBA-15 (H2O2) | 70 °C, 24 h | 22.6 | 100 | [50] |
| 5 | CoVSB-5 (H2O2) | 70 °C, 6 h | 57 | 75 | [51] |
| 6 | Ti-Fe-SBA-15 (H2O2) | 70 °C, 12 h | 37.1 | 86.3 | [49] |
| 7 | Ti-beta (H2O2) | 70 °C, 5 h | 20.1 | 57.2 | [45] |
| 8 | SiO2 (H2O2) | 50 °C, 6 h | - | - | [48] |
| 9 | 1.97% CeO2-SiO2 | 50 °C, 6 h | 42.9 | 42.90 | [48] |
| 10 | 0.98% CeO2-SiO2 | 50 °C, 12 h | 81.40 | 27.30 | [48] |
| 11 | Ni/SiO2 (H2O2) | 75 °C, 12 h | 31.2 | 90.20 | [52] |
| 12 | FePcS/NH2-MCM-48 | RT, 6 h | 21.9 | 23.90 | [53] |
| 13 | FePcS/NH2-MCM-48 | RT, 24 h | 65.5 | 21.4 | [53] |
| 14 | MIL@NTU-1 (TBHP) | 80 °C, 12 h | 31.4 | 12 | [54] |
| 15 | STP-1 (H2O2) | 70 °C, 10 h | 32.7 | 93.8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, R.; Chowdhury, A.; Bhattacharjee, S.; Bal, R.; Bhaumik, A. Selective Styrene Oxidation Catalyzed by Phosphate Modified Mesoporous Titanium Silicate. Chemistry 2023, 5, 589-601. https://doi.org/10.3390/chemistry5010042
Chatterjee R, Chowdhury A, Bhattacharjee S, Bal R, Bhaumik A. Selective Styrene Oxidation Catalyzed by Phosphate Modified Mesoporous Titanium Silicate. Chemistry. 2023; 5(1):589-601. https://doi.org/10.3390/chemistry5010042
Chicago/Turabian StyleChatterjee, Rupak, Avik Chowdhury, Sudip Bhattacharjee, Rajaram Bal, and Asim Bhaumik. 2023. "Selective Styrene Oxidation Catalyzed by Phosphate Modified Mesoporous Titanium Silicate" Chemistry 5, no. 1: 589-601. https://doi.org/10.3390/chemistry5010042
APA StyleChatterjee, R., Chowdhury, A., Bhattacharjee, S., Bal, R., & Bhaumik, A. (2023). Selective Styrene Oxidation Catalyzed by Phosphate Modified Mesoporous Titanium Silicate. Chemistry, 5(1), 589-601. https://doi.org/10.3390/chemistry5010042