Boranils: Versatile Multifunctional Organic Fluorophores for Innovative Applications
Abstract
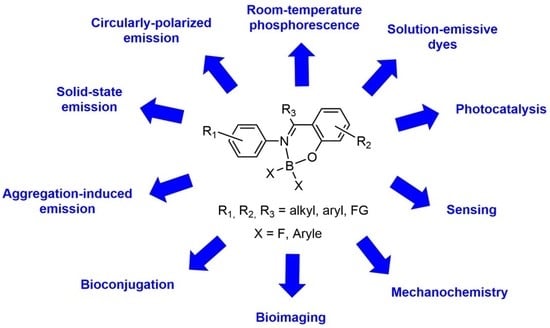
:1. Introduction
2. Synthesis, Derivatizations, and Applications
3. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Forrest, S.R.; Thompson, M.E. Organic Electronics and Optoelectronics. Chem. Rev. 2007, 107, 923. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234. [Google Scholar] [CrossRef]
- Gsänger, M.; Bialas, D.; Huang, L.; Stolte, M.; Würthner, F. Organic Semiconductors based on Dyes and Color Pigments. Adv. Mater. 2016, 28, 3615. [Google Scholar]
- Jun, J.V.; Chenoweth, D.M.; Petersson, E.J. Rational design of small molecule fluorescent probes for biological applications. Org. Biomol. Chem. 2020, 18, 5747. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kwon, N.; Lee, J.-H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143. [Google Scholar] [CrossRef]
- Singh, H.; Tiwari, K.; Tiwari, R.; Pramanik, S.K.; Das, A. Small Molecule as Fluorescent Probes for Monitoring Intracellular Enzymatic Transformations. Chem. Rev. 2019, 119, 11718. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028. [Google Scholar] [CrossRef] [PubMed]
- Wasielewski, M.R. Photoinduced electron transfer in supramolecular systems for artificial photosynthesis. Chem. Rev. 1992, 92, 435. [Google Scholar] [CrossRef]
- Lee, H.; Hong, K.-I.; Jang, W.-D. Design and applications of molecular probes containing porphyrin derivatives. Coord. Chem. Rev. 2018, 354, 46. [Google Scholar] [CrossRef]
- Tasior, M.; Kim, D.; Singha, S.; Krzeszewski, M.; Ahn, K.H.; Gryko, D.T. π-Expanded coumarins: Synthesis, optical properties and applications. J. Mater. Chem. C 2015, 3, 1421. [Google Scholar] [CrossRef]
- Beija, M.; Afonso, C.A.M.; Martinho, J.M.G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973. [Google Scholar] [CrossRef] [PubMed]
- Khopkar, S.; Shankarling, G. Synthesis, photophysical properties and applications of NIR absorbing unsymmetrical squaraines: A review. Dyes Pigm. 2019, 170, 107645. [Google Scholar] [CrossRef]
- Chen, L.; Lia, C.; Müllen, K. Beyond perylene diimides: Synthesis, assembly and function of higher rylene chromophores. J. Mater. Chem. C 2014, 2, 1938. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cao, Q.; Dan, F. Solid-Emissive BODIPY Derivatives: Design, Synthesis and Applications. Curr. Org. Chem. 2012, 16, 2970. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Boens, N.; Jiao, L.; Hao, E. Aromatic [b]-fused BODIPY dyes as promising near-infrared dyes. Org. Biomol. Chem. 2020, 18, 4135. [Google Scholar] [CrossRef]
- Ulrich, G.; Goze, C.; Guardigli, M.; Roda, A.; Ziessel, R. Pyrromethene Dialkynyl Borane Complexes for “Cascatelle” Energy Transfer and Protein Labeling. Angew. Chem. Int. Ed. 2005, 44, 3694. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited state intramolecular proton transfer (ESIPT): From principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials. Phys. Chem. Chem. Phys. 2012, 14, 8803. [Google Scholar] [CrossRef]
- Massue, J.; Jacquemin, D.; Ulrich, G. Molecular Engineering of Excited-state Intramolecular Proton Transfer (ESIPT) Dual and Triple Emitters. Chem. Lett. 2018, 47, 1083. [Google Scholar] [CrossRef]
- Frath, D.; Massue, J.; Ulrich, G.; Ziessel, R. Luminescent materials: Locking π-conjugated and heterocyclic ligands with boron(III). Angew. Chem. Int. Ed. 2014, 53, 2290. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewska, B.; Zakrzewska, A.; Mloston, G.; Budzák, S.; Mroczynska, K.; Grabarz, A.M.; Kaczorowska, M.A.; Jacquemin, D.; Osmiałowski, B. Synthesis and Photophysical Properties of Novel Donor–Acceptor N-(Pyridin-2-yl)-Substituted Benzo(thio)amides and Their Difluoroboranyl Derivatives. J. Phys. Chem. A 2016, 120, 4116. [Google Scholar] [CrossRef] [PubMed]
- Grabarz, A.M.; Laurent, A.D.; Jędrzejewska, B.; Zakrzewska, A.; Jacquemin, D.; Osmiałowski, B. The Influence of the π-Conjugated Spacer on Photophysical Properties of Difluoroboranyls Derived from Amides Carrying a Donor Group. J. Org. Chem. 2016, 81, 2280. [Google Scholar] [CrossRef] [PubMed]
- Massue, J.; Frath, D.; Ulrich, G.; Retailleau, P.; Ziessel, R. Synthesis of Luminescent 2-(2-Hydroxyphenyl)benzoxazole (HBO) Borate Complexes. Org. Lett. 2012, 14, 230. [Google Scholar] [CrossRef]
- Massue, J.; Frath, D.; Retailleau, P.; Ulrich, G.; Ziessel, R. Synthesis of Luminescent Ethynyl-Extended Regioisomers of Borate Complexes Based on 2-(2′-Hydroxyphenyl)benzoxazole. Chem. Eur. J. 2013, 19, 5375. [Google Scholar] [CrossRef]
- Benelhadj, K.; Massue, J.; Retailleau, P.; Ulrich, G.; Ziessel, R. 2-(2-Hydroxyphenyl)benzimidazole and 9,10-Phenanthroimidazole Chelates and Borate Complexes: Solution- and Solid-State Emitters. Org. Lett. 2013, 15, 2918. [Google Scholar] [CrossRef]
- Potopnyk, M.A.; Volyniuk, D.; Luboradzki, R.; Ceborska, M.; Hladka, I.; Danyliv, Y.; Grazulevicius, J.V. Organolithium-Mediated Postfunctionalization of Thiazolo[3,2- c][1,3,5,2]oxadiazaborinine Fluorescent Dyes. J. Org. Chem. 2020, 85, 6060. [Google Scholar] [CrossRef]
- Taguchi, J.; Matsuura, S.; Seki, T.; Ito, H. Synthesis and Tunable Optical Properties of C,N-Chelated Borate Luminophores Derived from Potassium Acyltrifluoroborates. Chem. Eur. J. 2020, 26, 2450. [Google Scholar] [CrossRef]
- Más-Montoya, M.; Montenegro, M.F.; Ferao, A.E.; Tárraga, A.; Rodríguez-López, J.N.; Curiel, D. Rigid π-Extended Boron Difluoride Complex with Mega-Stokes Shift for Bioimaging. Org. Lett. 2020, 22, 3356. [Google Scholar] [CrossRef]
- Frath, D.; Azizi, S.; Ulrich, G.; Retailleau, P.; Ziessel, R. Facile Synthesis of Highly Fluorescent Boranil Complexes. Org. Lett. 2011, 13, 3414. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, F.; Luo, H.; Liao, L.; Song, X.; Chen, W. Red-emitting boron difluoride complexes with a mega-large Stokes shift and unexpectedly high fluorescence quantum yield. Chem. Commun. 2020, 56, 2159. [Google Scholar] [CrossRef]
- Frath, D.; Didier, P.; Mély, Y.; Massue, J.; Ulrich, G. Vectorization and Intracellular Distribution of a Two-Photon-Absorbing, Near-Infrared-Emitting π-Extended Boranil Dye. ChemPhotoChem. 2017, 1, 109. [Google Scholar] [CrossRef]
- Benelhadj, K.; Massue, J.; Retailleau, P.; Chibani, S.; le Guennic, B.; Jacquemin, D.; Ziessel, R.; Ulrich, G. Solution- and Solid-State Luminescent Borate Complexes Based on a Substituted π-Conjugated 2-(6′-Hydroxy-5′-benzofuryl) Scaffold. Eur. J. Org. Chem. 2014, 2014, 7156–7164. [Google Scholar] [CrossRef]
- Urban, M.; Durka, K.; Jankowski, P.; Serwatowski, J.; Lulinski, S. Highly Fluorescent Red-Light Emitting Bis(boranils) Based on Naphthalene Backbone. J. Org. Chem. 2017, 82, 8234. [Google Scholar] [CrossRef]
- Frath, D.; Benelhadj, K.; Munch, M.; Massue, J.; Ulrich, G. Polyanils and Polyboranils: Synthesis, Optical Properties, and Aggregation-Induced Emission. J. Org. Chem. 2016, 81, 9658. [Google Scholar] [CrossRef]
- Crandall, L.A.; Dawadi, M.B.; Burrell, T.; Odoom, A.; Ziegler, C.J. Structure and electronics in dimeric boron π expanded azine and salphen complexes. Photochem. Photobiol. Sci. 2017, 16, 627. [Google Scholar] [CrossRef]
- Dobkowski, J.; Wnuk, P.; Buczyńska, J.; Pszona, M.; Orzanowska, G.; Frath, D.; Ulrich, G.; Massue, J.; Mosquera-Vázquez, S.; Vauthey, E.; et al. Substituent and Solvent Effects on the Excited State Deactivation Channels in Anils and Boranils. Chem. Eur. J. 2015, 21, 1312. [Google Scholar] [CrossRef]
- Nano, A.; Gullo, M.P.; Ventura, B.; Armaroli, N.; Barbieri, A.; Ziessel, R. Panchromatic luminescence from julolidine dyes exhibiting excited state intramolecular proton transfer. Chem. Commun. 2015, 51, 3351. [Google Scholar] [CrossRef]
- Suresh, D.; Gomes, C.S.B.; Lopes, P.S.; Figueira, C.A.; Ferreira, B.; Gomes, P.T.; di Paolo, R.E.; Maçanita, A.L.; Duarte, M.T.; Charas, A.; et al. Luminescent Di- and Trinuclear Boron Complexes Based on Aromatic Iminopyrrolyl Spacer Ligands: Synthesis, Characterization, and Application in OLEDs. Chem. Eur. J. 2015, 21, 9133. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Z.; Zhao, S.; Wang, Y.; Zhang, H. Diboron-containing fluorophores with extended ladder-type π-conjugated skeletons. Dalton Trans. 2011, 40, 1279. [Google Scholar] [CrossRef]
- Dhanunjayarao, K.; Mukundam, V.; Ramesh, M.; Venkatasubbaiah, K. Synthesis and Optical Properties of Salicylaldimine-Based Diboron Complexes. Eur. J. Inorg. Chem. 2014, 3, 539. [Google Scholar] [CrossRef]
- Wesela-Bauman, G.; Urban, M.; Lulinski, S.; Serwatowski, J.; Wozniak, K. Tuning of the colour and chemical stability of model boranils: A strong effect of structural modifications. Org. Biomol. Chem. 2015, 13, 3268. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Durka, K.; Gorka, P.; Wiosna-Salyga, G.; Nawara, K.; Jankowski, P.; Lulinski, S. The effect of locking π-conjugation in organoboron moieties in the structures of luminescent tetracoordinate boron complexes. Dalton Trans. 2019, 48, 8642. [Google Scholar] [CrossRef]
- Frath, D.; Azizi, S.; Ulrich, G.; Ziessel, R. Chemistry on Boranils: An Entry to Functionalized Fluorescent Dyes. Org. Lett. 2012, 14, 4774. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Ma, C.; Yang, W.; Yin, W.; Wei, J.; Li, N. Facile construction of boranil complexes with aggregation-induced emission characteristics and their specific lipid droplet imaging applications. Chem. Commun. 2019, 55, 8494. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, L.; Hao, Y.; Yue, X.; Cai, J.; Xiao, Q.; Huang, S.; Sheng, J.; Song, X. Detection of thiophenol in buffer, in serum, on filter paper strip, and in living cells using a red-emitting amino phenothiazine boranil based fluorescent probe with a large Stokes shift. Tetrahedron 2017, 73, 4529. [Google Scholar] [CrossRef]
- Zhu, D.; Yan, X.; Ren, A.; Cai, W.; Duan, Z.; Luo, Y. Modulation of ICT and PET processes in boranil derivatives: A ratiometric fluorescent probe for imaging of cysteine. Anal. Methods 2019, 11, 2579. [Google Scholar] [CrossRef]
- Shah, S.; Bajaj, A.; Shibu, A.; Ali, M.E.; Neelakandan, P.P. Iodo-Functionalized Salicylideneimine-Boron Complexes: Synthesis and Photosensitized Degradation of Organic Water Pollutants. Chem. Eur. J. 2018, 24, 18788. [Google Scholar] [CrossRef]
- Sun, T.; Cheng, D.; Chai, Y.; Gong, J.; Sun, M.; Zhao, F. High contrast mechanofluorochromic behavior of new tetraphenylethene-based Schiff base derivatives. Dyes Pigm. 2019, 170, 107619. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, R.; Yu, Q.; Zhang, X.; Wei, M.; Dai, Z. Boranil dyes bearing tetraphenylethene: Synthesis, AIE/AIEE effect properties, pH sensitive properties and application in live cell imaging. Tetrahedron Lett. 2018, 59, 2671. [Google Scholar] [CrossRef]
- Vaz, P.A.A.M.; Rocha, J.; Silva, A.M.S.; Guieu, S. Aggregation-induced emission enhancement of chiral boranils. New J. Chem. 2018, 42, 18166. [Google Scholar] [CrossRef]
- Nandi, R.P.; Sudhakar, P.; Kalluvettukuzhy, N.K.; Thilagar, P. Triarylborane-Appended Anils and Boranils: Solid-State Emission, Mechanofluorochromism, and Phosphorescence. Chem. Eur. J. 2020, 26, 16306. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Yang, H.; Sun, J.; Zhang, Z.; Sun, J.; Xue, P.; Lu, R. Salicylaldimine difluoroboron complexes containing tert-butyl groups: Nontraditional π-gelator and piezofluorochromic compounds. J. Mater. Chem. C 2015, 3, 10302. [Google Scholar] [CrossRef]
- Sun, J.; Yang, H.; Simalou, O.; Lv, K.; Zhai, L.; Zhao, J.; Lu, R. Mechanofluorochromic behaviors of triphenylamine functionalized salicylaldimine difluoroboron complexes. New J. Chem. 2019, 43, 10134. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Y.; Zhang, G.; Kong, L.; Yang, L.; Yang, J. Multi-stimuli-responsive fluorescence of a highly emissive difluoroboron complex in both solution and solid states. Cryst. Eng. Comm. 2017, 19, 1294. [Google Scholar] [CrossRef]
- Macé, A.; Hamrouni, K.; Gauthier, E.S.; Jean, M.; Vanthuyne, N.; Frédéric, L.; Pieters, G.; Caytan, E.; Roisnel, T.; Aloui, F.; et al. Circularly Polarized Fluorescent Helicene-Boranils: Synthesis, Photophysical and Chiroptical Properties. Chem. Eur. J. 2021, 27, 7959. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Perumal, K.; Blacque, O.; Garg, J.A.; Saiganesh, R.; Kabilan, S.; Balasubramanian, K.K.; Venkatesan, K. Metal-free triplet phosphors with high emission efficiency and high tenability. Angew. Chem. Int. Ed. 2014, 53, 6378. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, Y.; Xiao, L.; Chen, J.; Liao, Q.; Yao, J.; Fu, H. Organic Phosphorescence Nanowire Lasers. J. Am. Chem. Soc. 2017, 139, 6376. [Google Scholar] [CrossRef] [PubMed]
- Chibani, S.; Charaf-Eddin, A.; le Guennic, B.; Jacquemin, D. Boranil and related NBO Dyes: Insights from Theory. J. Chem. Theory Comput. 2013, 9, 3127. [Google Scholar] [CrossRef] [PubMed]
- Budzak, S.; Jacquemin, D. Excited state intramolecular proton transfer in julolidine derivatives: An ab initio study. Phys. Chem. Chem. Phys. 2018, 20, 25031. [Google Scholar] [CrossRef] [PubMed]
- Chaabene, M.; Agren, S.; Allouche, A.R.; Chaâbane, R.B.; Lahcinie, M.; Baouab, M.H.V. Theoretical and experimental investigations of complexation with BF3.Et2O effects on electronic structures, energies and photophysical properties of Anil and tetraphenyl (hydroxyl) imidazole. Appl. Organomet. Chem. 2019, 33, e5218. [Google Scholar] [CrossRef]
- Agren, S.; Chaabene, M.; Allouche, A.R.; Chaâbane, R.B.; Lahcinie, M.; Baouab, M.H.V. Blue Highly Fluorescent Boranil Derived from Anil Ligand: Synthesis, Characterization, Experimental and Theoretical Evaluation of Solvent Effect on Structures and Photophysical Properties. Appl. Organomet. Chem. 2020, 34, e5764. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massue, J.; Jacquemin, D.; Ulrich, G. Boranils: Versatile Multifunctional Organic Fluorophores for Innovative Applications. Organics 2021, 2, 365-375. https://doi.org/10.3390/org2040020
Massue J, Jacquemin D, Ulrich G. Boranils: Versatile Multifunctional Organic Fluorophores for Innovative Applications. Organics. 2021; 2(4):365-375. https://doi.org/10.3390/org2040020
Chicago/Turabian StyleMassue, Julien, Denis Jacquemin, and Gilles Ulrich. 2021. "Boranils: Versatile Multifunctional Organic Fluorophores for Innovative Applications" Organics 2, no. 4: 365-375. https://doi.org/10.3390/org2040020
APA StyleMassue, J., Jacquemin, D., & Ulrich, G. (2021). Boranils: Versatile Multifunctional Organic Fluorophores for Innovative Applications. Organics, 2(4), 365-375. https://doi.org/10.3390/org2040020