Synthesis of Novel Trisubstituted Olefin-Type Probe Molecules Containing N-Heterocycles and Their Application in Detection of Malononitrile
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Reaction Conditions
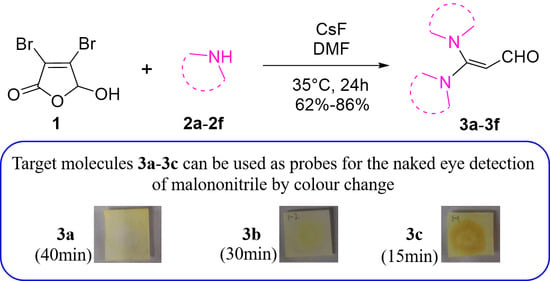
2.2. Investigation into the Range of Reaction Substrates
2.3. Structural Characterization Analysis
2.4. Mechanism Investigation
2.5. Application of 3a–3c in the Detection of Malononitrile
3. Materials and Methods
3.1. General Information
3.2. Experimental Procedure for the Synthesis of Compounds 3a–3f
3.3. Structural Characterization Data of Compounds 3a–3f
3.4. Preparation of the Test Strips and the Visual Detection towards Malononitrile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, Y.J.; Zhang, D.; Zhang, Y.X.; Liu, Y.L.; Cai, L.R.; Plaster, E.; Zheng, J. Fundamentals and exploration of aggregation-induced emission molecules for amyloid protein aggregation. J. Mater. Chem. B 2022, 10, 2280–2295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Zhang, L.L.; Zhao, Y.; Li, Y.J.; Shi, J.B.; Zhi, J.E.; Dong, Y.P. Helical self-assembly and Fe3+ detection of V-shaped AIE-active chiral tetraphenylbutadiene-based polyamides. Chem.-Eur. J. 2023, 29, e202301035. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.-H.; Hu, H.-R.; Liu, R.-B.; Sheng, G.-Z.; Niu, J.-J.; Fang, Y.; Wang, K.-P.; Hu, Z.-Q. A triphenylamine-based fluorescence probe for detection of hypochlorite in mitochondria. Spectrochim. Acta Part A 2023, 299, 122830. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-H.; Cao, X.-Y.; Chen, S.-H.; Yu, S.-W.; Lin, Y.-L.; Lin, S.-T.; Wang, Z.-Y. Design, synthesis and application of trisubstituted olefinic aggregation-induced emission molecules. Chin. J. Org. Chem. 2022, 42, 2355–2363. [Google Scholar] [CrossRef]
- Jia, Y.J.; Guo, S.S.; Han, Q.Z.; Zhu, J.L.; Zhang, X.L.; Na, N.; Ouyang, J. Target-triggered and controlled release plasmon- enhanced fluorescent AIE probe for conformational monitoring of insulin fibrillation. J. Mater. Chem. B 2021, 9, 5128–5135. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.C.; Gao, Y.; Pan, Q.Q.; Zhao, Z.W.; Wu, Y.; Zhao, L.; Geng, Y.; Su, Z.M. Whether the combination of AIE and TADF functional groups produces AIE-type TADF? A theoretical study on the synergistic effect of TPE and carbazole donor group/ thianthrene-tetraoxide acceptor group. Dyes Pigm. 2021, 194, 109547. [Google Scholar] [CrossRef]
- Chen, C.; Gao, H.Q.; Ou, H.L.; Kwok, R.T.K.; Tang, Y.H.; Zheng, D.H.; Ding, D. Amplification of activated near-infrared afterglow luminescence by introducing twisted molecular geometry for understanding neutrophil-involved diseases. J. Am. Chem. Soc. 2022, 144, 3429–3441. [Google Scholar] [CrossRef] [PubMed]
- Kachwal, V.; Laskar, I.R. Mechanofluorochromism with aggregation-induced emission (AIE) characteristics: A perspective applying isotropic and anisotropic force. Top. Curr. Chem. 2021, 379, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wolstenholme, C.H.; Carter, G.C.; Liu, H.B.; Hu, H.; Grainger, L.S.; Miao, K.; Fares, M.; Hoelzel, C.A.; Yennawar, H.P.; et al. Modulation of fluorescent protein chromophores to detect protein aggregation with turn-on fluorescence. J. Am. Chem. Soc. 2018, 140, 7381–7384. [Google Scholar] [CrossRef]
- Biesen, L.; May, L.; Nirmalananthan-Budau, N.; Hoffmann, K.; Resch-Genger, U.; Müller, T.J.J. Communication of bichromophore emission upon aggregation-Aroyl-S,N-ketene acetals as multifunctional sensor merocyanines. Chem. Eur. J. 2021, 27, 13426. [Google Scholar] [CrossRef]
- Wang, B.-W.; Jiang, K.; Li, J.-X.; Luo, S.-H.; Wang, Z.-Y.; Jiang, H.-F. 1,1-Diphenyl-vinylsulfide as a functional AIEgen derived from the aggregation-caused-quenching molecule 1,1-diphenylethene through simple thioetherification. Angew. Chem. Int. Ed. 2020, 59, 2338–2343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Wang, E.F.; Zhou, Y.; Zhang, L.; Chen, M.; Lin, X.R. A metal-free synthesis of 1,1-diphenylvinylsulfides with thiols via thioetherification under continuous-flow conditions. Org. Chem. Front. 2020, 7, 1490–1494. [Google Scholar] [CrossRef]
- Xiao, P.H.; Ma, K.; Kang, M.M.; Huang, L.Y.; Wu, Q.; Song, N.; Ge, J.Y.; Li, D.; Dong, J.X.; Wang, L.; et al. An aggregation-induced emission platform for efficient Golgi apparatus and endoplasmic reticulum specific imaging. Chem. Sci. 2021, 12, 13949–13957. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Navarro, A.; Garcia-Martinez, J.C. Styrylbenzene organogels and how the cyano groups tune the aggregation-induced emission. Dyes Pigm. 2021, 192, 109427. [Google Scholar] [CrossRef]
- Tian, J.W.; Teng, M.Z.; Song, M.; Li, Z.J.; Zhang, X.Y.; Xu, Y.Q. A feasible molecular engineering for bright π-conjugation free radical photosensitizers with aggregation-induced emission. Dyes Pigm. 2021, 194, 109651. [Google Scholar] [CrossRef]
- Liu, B.B.; He, W.; Lu, H.; Wang, K.; Huang, M.M.; Kwok, R.T.K.; Lam, J.W.Y.; Gao, L.C.; Yang, J.P.; Tang, B.Z. A facile design for multifunctional AIEgen based on tetraaniline derivatives. Sci. China Chem. 2019, 62, 732–738. [Google Scholar] [CrossRef]
- Jung, Y.; Park, N.K.; Kang, S.; Huh, Y.; Jung, J.; Hur, J.K.; Kim, D. Latent turn-on fluorescent probe for the detection of toxic malononitrile in water and its practical applications. Anal. Chim. Acta 2020, 1095, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sahu, B.; Das, U.K.; Patra, G.K. A reversible fluorescent-colorimetric malononitrile based novel Schiff-base chemosensor for visual detection of bicarbonate ion in aqueous solution. Inorg. Chim. Acta 2023, 552, 121491. [Google Scholar] [CrossRef]
- Li, M.X.; Gao, Y.; Zhang, Y.; Gong, S.; Tian, X.C.; Yang, Y.Q.; Xu, X.; Wang, Z.L.; Wang, S.F. A novel ratiometric fluorescent chemosensor for detecting malononitrile and application assisted with smartphone. Spectrochim. Acta Part A 2021, 262, 120135. [Google Scholar] [CrossRef] [PubMed]
- Karalliedde, L.; Wheeler, H.; Maclehose, R.; Murray, V. Possible immediate and long-term health effects following exposure to chemical warfare agents. Public Health 2000, 114, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.P.; Liu, J.; Zhang, Z.C.; Qi, Q.R.; Yao, S.; Huang, W.C. A Michael addition reaction-based fluorescent probe for malononitrile detection and its applications in aqueous solution, living cells and zebrafish. Analyst 2021, 146, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, P.; McCann, M.; Tubbs, N.; Judenherc-Haouzi, A.; Cheung, J.; Bouillaud, F. Antidotal effects of the phenothiazine chromophore methylene blue following cyanide Intoxication. Toxicol. Sci. 2019, 170, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Guo, X.J.; Teng, B.H.; Zhang, Q.; Zhang, P.; Ding, C.F. Ratiometric fluorescent strategy for malononitrile determination in organic and aqueous medium and biological imaging. Dyes Pigm. 2021, 184, 108859. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.Y.; Liu, C.Y.; Zhang, Y.; Su, M.J.; Rong, X.D.; Zhu, H.C.; Yu, M.H.; Sheng, W.L.; Zhu, B.C. Discovery of a highly selective and ultra-sensitive colorimetric fluorescent probe for malononitrile and its applications in living cells and zebrafish. New J. Chem. 2022, 46, 1713–1719. [Google Scholar] [CrossRef]
- Wang, W.F.; Luo, M.; Yao, W.W.; Ma, M.T.; Pullarkat, S.A.; Xu, L.; Leung, P.H. Catalyst-free and solvent-free cyanosilylation and Knoevenagel condensation of aldehydes. ACS Sustain. Chem. Eng. 2019, 7, 1718–1722. [Google Scholar] [CrossRef]
- Das, S.; Das, P.P.; Walton, J.W.; Quah, C.K.; Ghoshal, K.; Bhattacharyya, M. Aggregation-induced emission switch showing high contrast mehanofluorochromism and solvatofluorochromism: Specifically detects HSO3- over bar in bioimaging studies. Dyes Pigm. 2023, 217, 111413. [Google Scholar] [CrossRef]
- Pang, C.-M.; Cao, X.-Y.; Xiao, Y.; Luo, S.-H.; Chen, Q.; Zhou, Y.-J.; Wang, Z.-Y. N-alkylation briefly constructs tunable multifunctional sensor materials: Multianalyte detection and reversible adsorption. iScience 2021, 24, 103126. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; Huo, J.-P.; Cao, L.; Ding, S.; Wang, L.-Y.; Cao, D.-R.; Wang, Z.-Y. Design and application of tri-benzimidazolyl star-shape molecules as fluorescent chemosensors for the fast-response detection of fluoride ion. Sens. Actuators B 2016, 237, 865–871. [Google Scholar] [CrossRef]
- Chen, S.-H.; Jiang, K.; Liang, Y.-H.; He, J.-P.; Xu, B.-J.; Chen, Z.-H.; Wang, Z.-Y. Fine-tuning benzazole-based probe for the ultrasensitive detection of Hg2+ in water samples and seaweed samples. Food Chem. 2023, 428, 136800. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Chen, Z.-H.; Jiang, K.; Cao, X.-Y.; Chen, L.-Y.; Ouyang, J.; Wang, Z.-Y. Regulating donor-acceptor system toward highly efficient dual-state emission for sensitive response of nitroaromatic explosives. Spectrochim. Acta Part A 2023, 300, 122905. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-C.; You, J.-Y.; Jiang, K.; Wu, H.-Q.; Xiong, J.-F.; Wang, Z.-Y. Novel benzimidazole-based ratiometric fluorescent probes for acidic pH. Dyes Pigm. 2018, 149, 1–7. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Z.-X.; Zhou, Y.-J.; Chen, Q.; Yu, S.-W.; Luo, S.-H.; Wang, Z.-Y. Simple inorganic base promoted polycyclic construction using mucohalic acid as a C-3 synthon: Synthesis and AIE probe application of benzo[4,5]imidazo[1,2-a]pyri- dines. Org. Chem. Front. 2022, 9, 1127–1136. [Google Scholar] [CrossRef]
- Yu, S.L.; Hong, C.; Liu, Z.; Zhang, H. Cobalt-catalyzed vinylic C-H addition to formaldehyde: Synthesis of butenolides from acrylic acids and HCHO. Org. Lett. 2021, 23, 8359–8364. [Google Scholar] [CrossRef] [PubMed]
- Byczek-Wyrostek, A.; Kitel, R.; Rumak, K.; Skonieczna, M.; Kasprzycka, A.; Walczak, K. Simple 2(5H)-furanone derivatives with selective cytotoxicity towards non-small cell lung cancer cell line A549–synthesis, structure-activity relationship and biological evaluation. Eur. J. Med. Chem. 2018, 150, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Bailly, F.; Queffelec, C.; Mbemba, G.; Mouscadet, J.-F.; Pommery, N.; Pommery, J.; Henichart, J.-P.; Cotelle, P. Synthesis and biological activities of a series of 4, 5-diaryl-3-hydroxy-2(5H)-furanones. Eur. J. Med. Chem. 2008, 43, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Lyons, T.; Heras, V.L.; Recio, M.V.; Gahan, C.G.M.; O’Sullivan, T.P. Investigation of halogenated furanones as inhibitors of quorum sensing-regulated bioluminescence in Vibrio harveyi. Future Med. Chem. 2023, 15, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Valle-Amores, M.A.; Feberero, C.; Martin-Somer, A.; Díaz-Tendero, S.; Smith, A.D.; Fraile, A.; Alemán, J. Intramolecular hydrogen bond activation for kinetic resolution of furanone derivatives by an organocatalyzed [3+2] asymmetric cycloaddition. Org. Chem. Front. 2024, 11, 1028–1038. [Google Scholar] [CrossRef]
- Papidocha, S.M.; Bulthaupt, H.H.; Carreira, E.M. Synthesis of neocaesalpin A, AA, and nominal neocaesalpin K. Angew. Chem. Int. Ed. 2023, 62, e202310149. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Asami, T.; Sawa, A.; Uno, Y.; Hanada, M.; Taniyama, C.; Funakoshi, Y.; Masai, H.; Sawa, M. Discovery of novel furanone derivatives as potent Cdc7 kinase inhibitors. Eur. J. Med. Chem. 2017, 130, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Yu, Y.D.; Yu, Y.; Huang, F.; Baell, J.B. Copper-catalyzed olefinic C(sp2)-H activation/carbene insertion/ester hydrolysis/cyclization with aryl diazo esters for the synthesis of multisubstituted furanones. Adv. Synth. Catal. 2023, 365, 2601–2606. [Google Scholar] [CrossRef]
- Lepage, M.L.; Alachouzos, G.; Hermens, J.G.H.; Elders, N.; van den Berg, K.J.; Feringa, B.L. Electron-poor butenolides: The missing link between acrylates and maleic anhydride in radical polymerization. J. Am. Chem. Soc. 2023, 145, 17211–17219. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.-H.; Peng, X.-J.; Mo, Z.-Y.; Xu, Y.-L.; Tang, H.-T.; Wang, H.-S.; Sun, H.-B.; Pan, Y.-M. Transition-metal-free C-N and C-C formation: Synthesis of benzo[4,5] imidazo[1,2-a] pyridines and 2-pyridones from ynones. Green Chem. 2018, 20, 2007–2012. [Google Scholar] [CrossRef]
- Feng, B.-B.; Lu, L.; Li, C.; Wang, X.-S. Iodine-catalyzed synthesis of dibenzo[b,h][1,6]-naphthyridine-11-carboxamides via a domino reaction involving double elimination of hydrogen bromide. Org. Biomol. Chem. 2016, 14, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.G.V.; Bhaskar, R.; Harathi, J.; Jayaprakash, N. Selective colorimetric signaling of mercury (II) ions using a quinoline-based probe with INHIBIT logic gate behavior and test strip. Inorg. Chem. Commun. 2023, 148, 110364. [Google Scholar] [CrossRef]
- Ozturk, D.; Omeroglu, I.; Koksoy, B.; Gol, C.; Durmus, M. A BODIPY decorated multiple mode reusable paper-based colorimetric and fluorometric pH sensor. Dyes Pigm. 2022, 205, 110510. [Google Scholar] [CrossRef]







 | |||||
|---|---|---|---|---|---|
| Entry | Base (equiv.) | Temp. (°C) | Time (h) | Solvent | Yield of 3c (%) [b] |
| 1 | CsF (3.0) | 35 | 24 | DMF | 83 |
| 2 | NaOH (3.0) | 35 | 24 | DMF | 44 |
| 3 | Na2CO3 (3.0) | 35 | 24 | DMF | 55 |
| 4 | K2CO3 (3.0) | 35 | 24 | DMF | 46 |
| 5 | NaHCO3 (3.0) | 35 | 24 | DMF | 47 |
| 6 | CsF (4.0) | 35 | 24 | DMF | 85 |
| 7 | CsF (5.0) | 35 | 24 | DMF | 78 |
| 8 | CsF (4.0) | 35 | 24 | DMSO | 70 |
| 9 | CsF (4.0) | 35 | 24 | Dioxane | <10 |
| 10 | CsF (4.0) | 35 | 24 | Acetonitrile | 30 |
| 11 | CsF (4.0) | 35 | 24 | H2O | trace |
| 12 | CsF (4.0) | 35 | 24 | Toluene | 25 |
| 13 | CsF (4.0) | 35 | 24 | THF | 28 |
| 14 | CsF (4.0) | 25 | 24 | DMF | 78 |
| 15 | CsF (4.0) | 45 | 24 | DMF | 65 |
| 16 | CsF (4.0) | 55 | 24 | DMF | 45 |
| 17 | CsF (4.0) | 35 | 16 | DMF | 48 |
| 18 | CsF (4.0) | 35 | 20 | DMF | 63 |
| 19 | CsF (4.0) | 35 | 28 | DMF | 80 |
 |
 |
| Compound | 3c |
|---|---|
| Empirical formula | C13H16N4O |
| Formula weight | 244.30 |
| Temperature (K) | 293 (2) |
| Wavelength (Å) | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Unit cell dimensions (Å, °) | a = 9.848 (2), b = 9.0672 (16), c = 14.991 (3) |
| α = 90, β = 107.02 (2), γ = 90 | |
| Volume (Å3) | 1279.9 (5) |
| Z, Density (Mg/m3) | 4, 1.268 |
| F(000) | 520.0 |
| Theta range for data collection | 7.248 to 58.722° |
| Index ranges | −9 ≤ h ≤ 13, −12 ≤ k ≤ 12, −20 ≤ l ≤ 12 |
| Reflections collected | 5836 |
| Independent reflections | 2931 [R(int) = 0.0489, R(sigma) = 0.0896] |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2931/0/165 |
| Goodness-of-fit on F2 | 1.095 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0713, wR2 = 0.1296 |
| R indices (all data) | R1 = 0.1486, wR2 = 0.1749 |
| Largest diff. peak and hole | 0.17 and −0.25 e.Å−3 |
| Compound | Higher Concentration (10−1 M) Malononitrile Environment | Lower Concentration (10−3 M) Malononitrile Environment |
|---|---|---|
| 3a | 40 min | 200 min |
| 3b | 30 min | 180 min |
| 3c | 15 min | 60 min |
| 3d | No change | No change |
| 3e | No change | No change |
| 3f | No change | No change |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-H.; Yu, S.-W.; Xu, W.-J.; Li, M.-X.; Zeng, Y.; Deng, S.-W.; Lin, J.-Y.; Wang, Z.-Y. Synthesis of Novel Trisubstituted Olefin-Type Probe Molecules Containing N-Heterocycles and Their Application in Detection of Malononitrile. Organics 2024, 5, 46-58. https://doi.org/10.3390/org5020004
Chen Z-H, Yu S-W, Xu W-J, Li M-X, Zeng Y, Deng S-W, Lin J-Y, Wang Z-Y. Synthesis of Novel Trisubstituted Olefin-Type Probe Molecules Containing N-Heterocycles and Their Application in Detection of Malononitrile. Organics. 2024; 5(2):46-58. https://doi.org/10.3390/org5020004
Chicago/Turabian StyleChen, Zhao-Hua, Shi-Wei Yu, Wen-Jin Xu, Miao-Xin Li, Yong Zeng, Si-Wei Deng, Jian-Yun Lin, and Zhao-Yang Wang. 2024. "Synthesis of Novel Trisubstituted Olefin-Type Probe Molecules Containing N-Heterocycles and Their Application in Detection of Malononitrile" Organics 5, no. 2: 46-58. https://doi.org/10.3390/org5020004
APA StyleChen, Z. -H., Yu, S. -W., Xu, W. -J., Li, M. -X., Zeng, Y., Deng, S. -W., Lin, J. -Y., & Wang, Z. -Y. (2024). Synthesis of Novel Trisubstituted Olefin-Type Probe Molecules Containing N-Heterocycles and Their Application in Detection of Malononitrile. Organics, 5(2), 46-58. https://doi.org/10.3390/org5020004