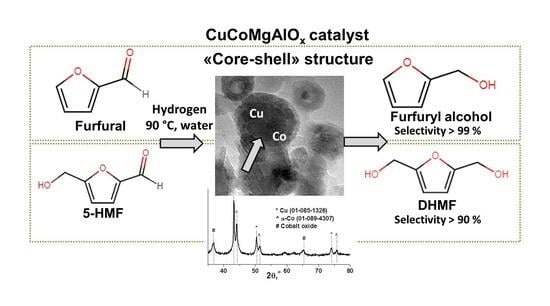
CuCoMgAlOx Mixed Oxides as Selective Catalysts for the Hydrogenation of Furan Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Physicochemical Characterization of the Samples
2.3. Catalyst Testing
3. Results
3.1. Study of Phase Transformations of the CuCoMgAl-Systems
3.2. Study of Catalytic Properties of CuCoMgAlOx Systems
3.3. Study of the Processes Occurred during Formation of Active form of the CuCoMgAlOx Catalyst
4. Conclusions
- In this study, CuCoMgAl-systems with LDH structure in a wide range of ratios (Co + Cu)/Mg = 0.5, 1, 2, and 3; and Co/Cu = 0.5, 1, and 2 were successfully prepared. The possibility of fine-tuning the properties of the samples by changing the ratios is shown.
- The value of the Co/Cu ratio has a significant impact on the formation of the oxide phase and the metal reduction process. An increase in the Co/Cu ratio in the composition of the systems contributed to the shift of the maxima of the decomposition temperatures of hydroxides and the reduction of metals (Cu, Co) to a higher temperature region, and, accordingly, an increase in thermal stability.
- Catalysts based on CuCoMgAl-layered hydroxides showed a high selectivity of carbonyl group hydrogenation in FAL and 5-HMF. The FOL selectivity for all studied systems was more than 99%, irrespective of their composition and the solvent used. The selectivity for DHMF was 95% on 5-HMF hydrogenation.
- The surfaces of the samples with different Co/Cu ratios (Co/Cu = 1 and 2) after calcination and reduction was the same and had a «core-shell» structure. «Core» consisted of copper and cobalt metallic particles with an average size of 12.7 nm. «Shell» consisted of CuCoMgAlOx non-stoichiometric spinel oxides. In the aqua phase FAL hydrogenation condition, the composition of the catalysts varied to a greater extent than their microstructures. The change in the phase composition also depended on the value of Co/Cu ratios. For the sample with Co/Cu = 1, the phase composition remained unchanged after the reaction. The decrease in the structural stability of the samples with the increase in Co/Cu ratios was observed (according to XRD data, the sample with Co/Cu = 2 was X-ray amorphous). The differences in the properties of the active phase, which is formed at the stage of high-temperature treatments, means that even a small change in the ratios (Co/Cu = 1 and 2) contributed to a change in thermal stability, completeness, and the ease of the reduction of metals, as well as to different phase composition after the reaction.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chien Truong, C.; Kumar Mishra, D.; Hyeok Ko, S.; Jin Kim, Y.; Suh, Y.W. Sustainable Catalytic Transformation of Biomass-Derived 5-Hydroxymethylfurfural to 2, 5-Bis (hydroxymethyl) tetrahydrofuran. ChemSusChem 2022, 15, e202200178. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. A versatile bi-metallic copper–cobalt catalyst for liquid phase hydrogenation of furfural to 2-methylfuran. RSC Adv. 2016, 6, 1649–1658. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Kong, X.; Zhu, Y. Catalytic Conversion of 5-Hydroxymethylfurfural to High-Value Derivatives by Selective Activation of C−O, C=O, and C=C Bonds. ChemSusChem 2022, 15, e202200421. [Google Scholar] [CrossRef]
- Xu, W.; Xia, Q.; Zhang, Y.; Guo, Y.; Wang, Y.; Lu, G. Effective production of octane from biomass derivatives under mild conditions. ChemSusChem 2011, 4, 1758–1761. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef]
- Kazi, F.K.; Patel, A.D.; Serrano-Ruiz, J.C.; Dumesic, J.A.; Anex, R.P. Techno-economic analysis of dimethylfuran (DMF) and hydroxymethylfurfural (HMF) production from pure fructose in catalytic processes. Chem. Eng. J. 2011, 169, 329–338. [Google Scholar] [CrossRef]
- Singh, G.; Singh, L.; Gahtori, J.; Gupta, R.K.; Samanta, C.; Bal, R.; Bordoloi, A. Catalytic hydrogenation of furfural to furfuryl alcohol over chromium-free catalyst: Enhanced selectivity in the presence of solvent. Mol. Catal. 2021, 500, 111339. [Google Scholar] [CrossRef]
- Manikandan, M.; Venugopal, A.K.; Nagpure, A.S.; Chilukuri, S.; Raja, T. Promotional effect of Fe on the performance of supported Cu catalyst for ambient pressure hydrogenation of furfural. RSC Adv. 2016, 6, 3888–3898. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, D.; van Haandel, L.; Ye, F.; Xue, T.; Hensen, E.J.; Guan, Y. Selective liquid phase hydrogenation of furfural to furfuryl alcohol by Ru/Zr-MOFs. J. Mol. Catal. A–Chem. 2015, 406, 58–64. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Lin, Y.; Liang, Y.; Chen, Y.; Zhou, W. Catalytic hydrogenation of 5-hydroxymethylfurfural to 2, 5-dimethylfuran over Ru based catalyst: Effects of process parameters on conversion and products selectivity. Renew. Energy 2020, 160, 261–268. [Google Scholar] [CrossRef]
- Silva, W.R.; Matsubara, E.Y.; Rosolen, J.M.; Donate, P.M.; Gunnella, R. Pd catalysts supported on different hydrophilic or hydrophobic carbonaceous substrate for furfural and 5-(hydroxymethyl)-furfural hydrogenation in water. Mol. Catal. 2021, 504, 111496. [Google Scholar] [CrossRef]
- Byun, M.Y.; Park, D.W.; Lee, M.S. Effect of oxide supports on the activity of Pd based catalysts for furfural hydrogenation. Catalysts 2020, 10, 837. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Talsi, V.P.; Gulyaeva, T.I.; Trenikhin, M.V.; Belskaya, O.B. Aqueous-phase hydrogenation of furfural over supported palladium catalysts: Effect of the support on the reaction routes. React. Kinet. Mech. Catal. 2019, 126, 811–827. [Google Scholar] [CrossRef]
- Merlo, A.B.; Vetere, V.; Ruggera, J.F.; Casella, M.L. Bimetallic PtSn catalyst for the selective hydrogenation of furfural to furfuryl alcohol in liquid-phase. Catal. Commun. 2009, 10, 1665–1669. [Google Scholar] [CrossRef]
- Redina, E.A.; Vikanova, K.V.; Kapustin, G.I. Monometallic Copper Catalysts for the Hydrogenation of 5-Hydroxymethylfurfural. Russ. J. Phys. Chem. A 2020, 94, 2558–2562. [Google Scholar] [CrossRef]
- Endot, N.A.; Junid, R.; Jamil MS, S. Insight into biomass upgrade: A review on hydrogenation of 5-Hydroxymethylfurfural (HMF) to 2, 5-Dimethylfuran (DMF). Molecules 2021, 26, 6848. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Y.; Hu, D.; Guo, R.; Yan, K.; Luque, R. Chemical transformations of 5-hydroxymethylfurfural to highly added value products: Present and future. Green Chem. 2023, 25, 871–892. [Google Scholar] [CrossRef]
- Yang, P.; Cui, Q.; Zu, Y.; Liu, X.; Lu, G.; Wang, Y. Catalytic production of 2, 5-dimethylfuran from 5-hydroxymethylfurfural over Ni/Co3O4 catalyst. Catal. Commun. 2015, 66, 55–59. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Lee, H.; Lee, J.; Kwak, J.H.; An, K. Mesoporous mixed CuCo oxides as robust catalysts for liquid-phase furfural hydrogenation. Appl. Catal. A–Gen. 2019, 571, 118–126. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Huang, Z.; Yuan, G. Carbon-coated Cu-Co bimetallic nanoparticles as selective and recyclable catalysts for production of biofuel 2, 5-dimethylfuran. Appl. Catal. B–Environ. 2017, 200, 192–199. [Google Scholar] [CrossRef]
- Mascolo, G.; Mascolo, M.C. On the synthesis of layered double hydroxides (LDHs) by reconstruction method based on the “memory effect”. Microporous Mesoporous Mater. 2015, 214, 246–248. [Google Scholar] [CrossRef]
- Sun, K.; Liu, Z.; Song, S.; Liu, W.; Wang, P.; Zhang, T.; Xue, Y.; Wang, Y.; Tan, Y. Effect of hydroxyl groups on CuCoMg nanosheets for ethanol and higher alcohol synthesis from syngas. Ind. Eng. Chem. Res. 2021, 60, 2388–2399. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Vasilevich, A.V.; Leont’eva, N.N.; Baklanova, O.N.; Likholobov, V.A. Effect of the composition of initial components and the conditions of activation on the mechanochemical synthesis of magnesium–aluminum layered double hydroxides. Kinet. Catal. 2018, 59, 521–531. [Google Scholar] [CrossRef]
- Lee, S.B.; Ko, E.H.; Park, J.Y.; Oh, J.M. Mixed metal oxide by calcination of layered double hydroxide: Parameters affecting specific surface area. Nanomaterials 2021, 11, 1153. [Google Scholar] [CrossRef]
- Bukhtiyarova, M.V. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2018, 269, 494–506. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Likholobov, V.A. Effect of the nature of the active-component precursor on the properties of Pt/MgAlOx catalysts in propane and n-decane dehydrogenation. Kinet. Catal. 2017, 58, 383–391. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Mironenko, R.M.; Kobzar, E.O.; Leont’eva, N.N.; Gulyaeva, T.I.; Vasilevich, A.V.; Serkova, A.N.; Salanov, A.N.; Lavrenov, A.V. Synthesis of CuAl-, CoAl-, and CuCoAl-Catalysts from Layered Hydroxides for Furfural Hydrogenation. Eng 2022, 3, 400–411. [Google Scholar] [CrossRef]
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Catal. B–Environ. 2016, 180, 580–585. [Google Scholar] [CrossRef]
- Singh, U.K.; Vannice, M.A. Kinetics of liquid-phase hydrogenation reactions over supported metal catalysts—A review. Appl. Catal. A–Gen. 2001, 213, 1–24. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.; Zheng, L.; Wang, B.; Bi, R.; He, Y.; Liu, H.; Li, D. Interfacial structure-determined reaction pathway and selectivity for 5-(hydroxymethyl) furfural hydrogenation over Cu-based catalysts. ACS Catal. 2019, 10, 1353–1365. [Google Scholar] [CrossRef]
- Tan, X.Y.; Ungur, L.; Chin, W.S. Carbonate-free CoAl layered double hydroxides supercapacitors: Controlled precipitation via acid mediated decomplexation. Appl. Clay Sci. 2022, 224, 106519. [Google Scholar] [CrossRef]
- Kobzar, E.O.; Stepanova, L.N.; Leont’eva, N.N.; Gulyaeva, T.I.; Trenikhin, M.V.; Lavrenov, A.V. Effect of the composition and synthesis procedure of catalysts based on CoAl hydroxides on their properties in furfural hydrogenation. Kinet. Catal. 2023, 64, 473–483. [Google Scholar] [CrossRef]
- Arnoldy, P.; Moulijn, J.A. Temperature-programmed reduction of CoOAl2O3 catalysts. J. Catal. 1985, 93, 38–54. [Google Scholar] [CrossRef]
- Ribet, S.; Tichit, D.; Coq, B.; Ducourant, B.; Morato, F. Synthesis and activation of Co–Mg–Al layered double hydroxides. J. Solid State Chem. 1999, 142, 382–392. [Google Scholar] [CrossRef]







| Sample with (Co + Cu)/Mg | c, Å | a, Å | Lc, Å | La, Å |
|---|---|---|---|---|
| Lattice parameters for samples with Co/Cu = 0.5 | ||||
| 0.5 | 22.88 | 3.052 | 95 | 167 |
| 1 | 22.87 | 3.055 | 89 | 138 |
| 2 | 22.86 | 3.061 | 97 | 138 |
| 3 | 22.72 | 3.060 | 109 | 138 |
| Lattice parameters for samples with Co/Cu = 1 | ||||
| 0.5 | 22.86 | 3.052 | 105 | 169 |
| 1 | 22.88 | 3.056 | 101 | 181 |
| 2 | 22.79 | 3.059 | 88 | 160 |
| 3 | 22.82 | 3.062 | 91 | 148 |
| Lattice parameters for samples with Co/Cu = 2 | ||||
| 0.5 | 22.87 | 3.051 | 76 | 142 |
| 1 | 22.80 | 3.056 | 84 | 121 |
| 2 | 22.82 | 3.058 | 98 | 166 |
| 3 | 22.76 | 3.060 | 105 | 181 |
| Sample ** | 5-HMF Conversion, % | Selectivity to DHMF, % | Yield of DHMF, % |
|---|---|---|---|
| Co/Cu = 1 | 73 | 95 | 69 |
| Co/Cu = 2 | 74 | 90 | 67 |
| Sample * | TMax, °C | nH2, mmol/g |
|---|---|---|
| Co/Cu = 1 | 171 ~480 | 4.62 3.13 |
| Co/Cu = 2 | 174 ~500 | 3.88 4.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobzar, E.O.; Stepanova, L.N.; Nepomniashchii, A.A.; Vasilevich, A.V.; Gulyaeva, T.I.; Trenikhin, M.V.; Lavrenov, A.V. CuCoMgAlOx Mixed Oxides as Selective Catalysts for the Hydrogenation of Furan Compounds. Hydrogen 2023, 4, 644-657. https://doi.org/10.3390/hydrogen4030041
Kobzar EO, Stepanova LN, Nepomniashchii AA, Vasilevich AV, Gulyaeva TI, Trenikhin MV, Lavrenov AV. CuCoMgAlOx Mixed Oxides as Selective Catalysts for the Hydrogenation of Furan Compounds. Hydrogen. 2023; 4(3):644-657. https://doi.org/10.3390/hydrogen4030041
Chicago/Turabian StyleKobzar, Elena O., Liudmila N. Stepanova, Aleksandr A. Nepomniashchii, Anastasia V. Vasilevich, Tatiana I. Gulyaeva, Mikhail V. Trenikhin, and Aleksandr V. Lavrenov. 2023. "CuCoMgAlOx Mixed Oxides as Selective Catalysts for the Hydrogenation of Furan Compounds" Hydrogen 4, no. 3: 644-657. https://doi.org/10.3390/hydrogen4030041
APA StyleKobzar, E. O., Stepanova, L. N., Nepomniashchii, A. A., Vasilevich, A. V., Gulyaeva, T. I., Trenikhin, M. V., & Lavrenov, A. V. (2023). CuCoMgAlOx Mixed Oxides as Selective Catalysts for the Hydrogenation of Furan Compounds. Hydrogen, 4(3), 644-657. https://doi.org/10.3390/hydrogen4030041