The Impact of Moderate-to-High-Intensity Exercise Protocols on Glycated Hemoglobin Levels in Type 2 Diabetes Patients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Identifying Eligible Studies
3.2. General Characteristics of Studies
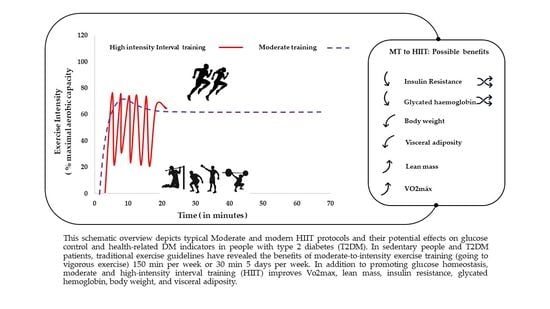
3.3. Main Findings of the Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Fronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Rifai, R.H.; Majeed, M.; Qambar, M.A.; Ibrahim, A.; AlYammahi, K.M.; Aziz, F. Type 2 diabetes and pre-diabetes mellitus: A systematic review and meta-analysis of prevalence studies in women of childbearing age in the Middle East and North Africa, 2000–2018. Syst. Rev. 2019, 8, 268. [Google Scholar] [CrossRef]
- Clinic, C. Type 2 Diabetes. 2021. Available online: https://my.clevelandclinic.org/health/diseases/21501-type-2-diabetes (accessed on 12 December 2022).
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Chew, B.H.; Vos, R.C.; Metzendorf, M.I.; Scholten, R.J.; Rutten, G.E. Psychological interventions for diabetes-related distress in adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2017, 9. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Santos, M.; West, E.; Skali, H.; Forman, D.E.; Nadruz, W.J.; Shah, A.M. Resting Heart Rate and Chronotropic Response to Exercise: Prognostic Implications in Heart Failure Across the Left Ventricular Ejection Fraction Spectrum. J. Card Fail. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Buckingham, B.; Christiansen, J.S.; Montori, V.M.; Tamborlane, W.V.; Vigersky, R.A.; Wolpert, H. Withdrawn as duplicate: Corrigendum to: Continuous Glucose Monitoring: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2022, 107, e2220. [Google Scholar]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2018, 12, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Niwaha, A.J.; Rodgers, L.R.; Greiner, R.; Balungi, P.A.; Mwebaze, R.; McDonald, T.J.; Hattersley, A.T.; Shields, B.M.; Nyirenda, M.J.; Jones, A.G. HbA1c performs well in monitoring glucose control even in populations with high prevalence of medical conditions that may alter its reliability: The OPTIMAL observational multicenter study. BMJ Open Diabetes Res. Care 2021, 9, e002350. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.J.; Smiles, W.; Hawley, J.A. The relationship between exercise, nutrition and type 2 diabetes. Med. Sport Sci. 2014, 60, 1–10. [Google Scholar] [PubMed]
- Li, D.-D.; Yang, Y.; Gao, Z.-Y.; Zhao, L.-H.; Yang, X.; Xu, F.; Yu, C.; Zhang, X.-L.; Wang, X.-Q.; Wang, L.-H.; et al. Sedentary lifestyle and body composition in type 2 diabetes. Diabetol. Metab. Syndr. 2022, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.K.D.; Nunes, F.; Queiros, V.S.; Cobucci, R.N.; Dantas, P.B.; Soares, G.M.; Cabral, B.; Maranhão, T.M.O.; Dantas, P.M.S. Effect of high-intensity interval training on metabolic parameters in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2021, 16, e0245023. [Google Scholar] [CrossRef]
- Younk, L.M.; Mikeladze, M.; Tate, D.; Davis, S.N. Exercise-related hypoglycemia in diabetes mellitus. Expert. Rev. Endocrinol. Metab. 2011, 6, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Francois, M.E.; Little, J.P. Effectiveness and safety of high-intensity interval training in patients with type 2 diabetes. Diabetes Spectr. 2015, 28, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Maldonado, A.; García-Suárez, P.C.; Rentería, I.; Moncada-Jiménez, J.; Plaisance, E.P. Impact of high-intensity interval training and sprint interval training on peripheral markers of glycemic control in metabolic syndrome and type 2 diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165820. [Google Scholar] [CrossRef]
- Amanat, S.; Ghahri, S.; Dianatinasab, A.; Fararouei, M.; Dianatinasab, M. Exercise and Type 2 Diabetes. Adv. Exp. Med. Biol. 2020, 1228, 91–105. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P. Evidence informing practice: Introducing the mini-review. Br. J. Community Nurs. 2002, 7, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.; Vaidya, V.; Houghton, D.; Zalewski, P.; Seferovic, J.P.; Hallsworth, K.; MacGowan, G.A.; Trenell, M.I.; Jakovljevic, D.G. Unsupervised high-intensity interval training improves glycaemic control but not cardiovascular autonomic function in type 2 diabetes patients: A randomised controlled trial. Diab. Vasc. Dis. Res. 2019, 16, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hangping, Z.; Xiaona, Q.; Qi, Z.; Qingchun, L.; Na, Y.; Lijin, J.; Siying, L.; Shuo, Z.; Xiaoming, Z.; Xiaoxia, L.; et al. The impact on glycemic control through progressive resistance training with bioDensity(TM) in Chinese elderly patients with type 2 diabetes: The PReTTy2 (Progressive Resistance Training in Type 2 Diabetes) Trial. Diabetes Res. Clin. Pract. 2019, 150, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.Y.; Karstoft, K.; MacDonald, C.S.; Hansen, K.B.; Ellingsgaard, H.; Hartmann, B.; Wewer Albrechtsen, N.J.; Vaag, A.A.; Holst, J.J.; Pedersen, B.K.; et al. Effects of an intensive lifestyle intervention on the underlying mechanisms of improved glycaemic control in individuals with type 2 diabetes: A secondary analysis of a randomised clinical trial. Diabetologia 2020, 63, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.P.; Júdice, P.B.; Ribeiro, R.; Andrade, R.; Raposo, J.; Dores, H.; Bicho, M.; Sardinha, L.B. Effectiveness of high-intensity interval training combined with resistance training versus continuous moderate-intensity training combined with resistance training in patients with type 2 diabetes: A one-year randomized controlled trial. Diabetes Obes. Metab. 2019, 21, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Björnholm, M.; Fritz, T.; Krook, A.; Zierath, J.R.; Wallberg-Henriksson, H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: A randomised crossover trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Verboven, K.; Wens, I.; Vandenabeele, F.; Stevens, A.N.; Celie, B.; Lapauw, B.; Dendale, P.; Van Loon, L.J.C.; Calders, P.; Hansen, D. Impact of Exercise-Nutritional State Interactions in Patients with Type 2 Diabetes. Med. Sci. Sports Exerc. 2020, 52, 720–728. [Google Scholar] [CrossRef]
- Winding, K.M.; Munch, G.W.; Iepsen, U.W.; Van Hall, G.; Pedersen, B.K.; Mortensen, S.P. The effect on glycaemic control of low-volume high-intensity interval training versus endurance training in individuals with type 2 diabetes. Diabetes Obes. Metab. 2018, 20, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; Nielsen, J.S.; Thomsen, C.; Pedersen, B.K.; Solomon, T.P. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: A randomized, controlled trial. Diabetes Care 2013, 36, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsisia, H.F.; Aneisb, Y.M.; Mounirc, K.M. Impact of high-intensity interval training on HbA1c in patients with type 2 diabetes mellitus. Bull. Fac. Phys. Ther. 2015, 20, 168–175. [Google Scholar] [CrossRef]
- Cassidy, S.; Thoma, C.; Houghton, D.; Trenell, M.I. High-intensity interval training: A review of its impact on glucose control and cardiometabolic health. Diabetologia 2017, 60, 7–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Mendes, R.; Sousa, N.; Themudo-Barata, J.L.; Reis, V.M. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control. Int. J. Env. Res. Public. Health 2019, 16, 4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mello, M.B.; Righi, N.C.; Schuch, F.B.; Signori, L.U.; da Silva, A.M.V. Effect of high-intensity interval training protocols on VO2max and HbA1c level in people with type 2 diabetes: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101586. [Google Scholar] [CrossRef] [PubMed]
| Acronym | Information | Concepts | MeSH Terms |
|---|---|---|---|
| P | T2DM patients | People diagnosed with type II diabetes, which causes insulin resistance, among other symptoms | “Diabetes Mellitus, Type 2” OR/AND “Type 2 Diabetes” |
| I | HIIT | A type of exercise proocol that involves moderate continuos and/or alternating between periods of vigorous exercise and rest or recovery | “exercise” OR/AND “exercise therapy” OR/AND “moderate exercise” OR/AND “High Intensity Interval training” |
| C | T2DM patients | Without a specific treatment and/or involving isolated or combined physical exercise. | - |
| O | Hb1C | Reflects erythrocytes’ cumulative glucose exposure over a time period proportional to erythrocyte survival. | “Glycated hemoglobin” AND Glycated human hemoglobin” |
| Author | Participants (Mean Age) | Description of Interventions | Comparison (Controls) | Outcome (HbA1c/mmol/mol) |
|---|---|---|---|---|
| Cassidy, S. et al. 2019 [25] | n = 22 60 years old | 12 weeks, HIIT group: 36 cycle sessions (3 sessions/week). 1 week was 2 min, increasing 10 s each week. | No Exercise | Decreased on HIIT Group on HbA1c 54.4 ± 3.3 vs. 51.6 ± 3.2 compared no exercise group 55.0 ± 1.8 57.0 ± 2.3. |
| Hangping, Z. et al. 2019 [26] | n = 265 66 years old | 1 training session/week, 5–10 min weekly, 4 exercises. | No Exercise | PRT protocol was more efficient 6.83 ± 1.31vs 6.75 ± 0.93, than no exercise group 6.92 ± 1. Vs. 6.85 ± 1.17. |
| Johansen, M. et al. 2020 [27] | n = 95 56 years old | 12 months 5 or 6 aerobic exercise sessions/week, 2 or 3 sessions/week, combined with resistance exercise. | No Exercise | BG reduced in the END + RT, 48.7 (9.0) vs −3.3 (−5.0, −1.7) compared with the standard care group, 50.2 (9.6). vs. −0.5 (−2.7, 1.8). |
| Magalhães, J. et al. 2018 [28] | n = 80 59 years old | 12 months, MCT–continuous cycling at 40 to 60% HRR, HIIT group, both groups complete RT 10–12 repetitions. | HIIT RT MCT RT | No interaction between the intervention groups, HIIT + RT Group 52.1 ± 9.6 vs. 52.8 ± 7.1, MCT + RCT Group 53.0 ± 17.4. vs. 54.0 ± 14.8, and control group 51.7 ± 11.7 vs. 54.8 ± 11.1, in glycemic variables. |
| Savikj, M. et al. 2019 [29] | n = 11 60 years old | Cycle ergometer: 7 min warm-up, 6 pulses of 1 min (220 W, range 180–350 W) 75 rpm. 2 weeks, 3 sessions/week. | Post-morning Post-afternoon | Afternoon HIIT 48.3 ± 3.9 vs. 46.1 ± 2.7, was better at improving BG, but post-Morning 48.3 ± 3.9 vs. 45.1 ± 2.1, was better on HbA1c reduction in post-morning intervention. |
| Verboven, K. et al. 2020 [30] | n = 25 61 years old | 12-week endurance training (3 exercise sessions/week), 25 min—walking, 20 min cycling, 65% of baseline VO2peak. | Fasted state Fed state | HbA1c better improved with exercise performed in the postprandial period 6.6 [6.3–7.5] vs. 6.3 [6.0–6.9], than 7.4 [6.8–8.2] vs. 7.7 [6.7–8.3]. |
| Winding, K. et al. 2018 [31] | n = 29 56 years old | 11-week, on bicycle, 3 days/week. END—40 min/session at 50% of Wpeak, group. HIIT—20 min/session of 95% Wpeak. | END HIIT | HIIT Intervention 7.4 [6.8–8.2] vs 7.7 [6.7–8.3] a statistically significant difference was observed in the reduction of HbA1c, when compare bout group, END group 52.2 ± 10.1. vs 51.4 ± 8.8 and, no exercise group 53.2 ± 12.6 vs 51.8 ± 11.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, A.; Furtado, G.; de Barros, M.P.; Bachi, A.L.L.; Ferreira, J.P.; Sardão, V.A.; Rama, L.; Teixeira, A. The Impact of Moderate-to-High-Intensity Exercise Protocols on Glycated Hemoglobin Levels in Type 2 Diabetes Patients. Diabetology 2023, 4, 11-18. https://doi.org/10.3390/diabetology4010002
Pedrosa A, Furtado G, de Barros MP, Bachi ALL, Ferreira JP, Sardão VA, Rama L, Teixeira A. The Impact of Moderate-to-High-Intensity Exercise Protocols on Glycated Hemoglobin Levels in Type 2 Diabetes Patients. Diabetology. 2023; 4(1):11-18. https://doi.org/10.3390/diabetology4010002
Chicago/Turabian StylePedrosa, Ana, Guilherme Furtado, Marcelo Paes de Barros, André Luís Lacerda Bachi, José Pedro Ferreira, Vilma A. Sardão, Luís Rama, and Ana Teixeira. 2023. "The Impact of Moderate-to-High-Intensity Exercise Protocols on Glycated Hemoglobin Levels in Type 2 Diabetes Patients" Diabetology 4, no. 1: 11-18. https://doi.org/10.3390/diabetology4010002
APA StylePedrosa, A., Furtado, G., de Barros, M. P., Bachi, A. L. L., Ferreira, J. P., Sardão, V. A., Rama, L., & Teixeira, A. (2023). The Impact of Moderate-to-High-Intensity Exercise Protocols on Glycated Hemoglobin Levels in Type 2 Diabetes Patients. Diabetology, 4(1), 11-18. https://doi.org/10.3390/diabetology4010002