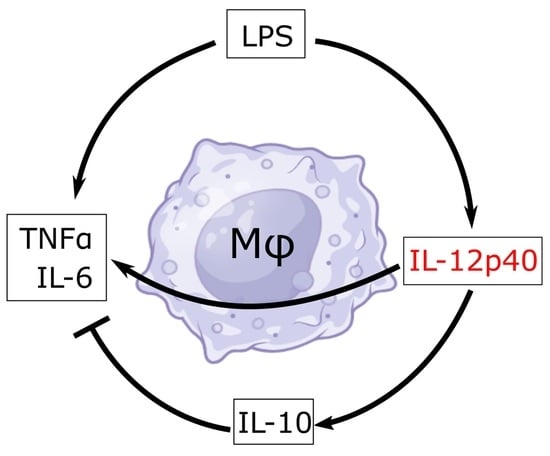
IL-12p40 Monomer: A Potential Player in Macrophage Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Culturing of RAW 264.7 Macrophage Cells
2.3. Isolation of Primary Lung Macrophages
2.4. ELISA
2.5. Immunofluorescence
2.6. Intranasal Administration of Recombinant p40 Monomer
2.7. RNA Extraction and Quantitative Real-Time PCR (qPCR)
2.8. FACS Analysis
2.9. Immunoblotting Analysis
2.10. Statistical Analysis
3. Results
3.1. Recombinant p40 Monomer Treatment Induced the Expression of Anti-Inflammatory Cytokine IL-10, Pro-Inflammatory Cytokines, and Different Immunomodulatory Molecules in RAW 264.7 Macrophages
3.2. Treatment of Primary Murine Lung Macrophages with Recombinant p40 Monomer Induced Similar Pro- and Anti-Inflammatory Phenotypes in RAW 264.7 Cells
3.3. Recombinant p40 Monomer Attenuated LPS-Driven M1 Polarization in RAW 264.7 Cells and Primary Murine Lung Macrophages
3.4. mAb-Mediated Neutralization of p40 Monomer Resulted in Higher Levels of Pro-Inflammatory Cytokine Release and iNOS Expression in LPS-Treated RAW 264.7 Cells
3.5. Intranasal Administration of Recombinant p40 Monomer Resulted in Increased Levels of CD68+TNFα+IL-10+ Macrophages In Vivo in the Lungs of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity—Basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Mondal, S.; Kundu, M.; Jana, M.; Roy, A.; Rangasamy, S.B.; Modi, K.K.; Wallace, J.; Albalawi, Y.A.; Balabanov, R.; Pahan, K. IL-12 p40 monomer is different from other IL-12 family members to selectively inhibit IL-12Rbeta1 internalization and suppress EAE. Proc. Natl. Acad. Sci. USA 2020, 117, 21557–21567. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Zhang, S.-X.; Wu, H.-J.; Rong, X.-L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, E.N. Inflammatory cytokines within the central nervous system: Sources, function, and mechanism of action. Am. J. Physiol. 1992, 263, C1–C16. [Google Scholar] [CrossRef]
- Gately, M.K.; Renzetti, L.M.; Magram, J.; Stern, A.S.; Adorini, L.; Gubler, U.; Presky, D.H. The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998, 16, 495–521. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Carvajal, D.; Ling, P.; Podlaski, F.J.; Stremlo, D.L.; Familletti, P.C.; Gubler, U.; Presky, D.H.; Stern, A.S.; Gately, M.K. Mouse interleukin-12 (IL-12) p40 homodimer: A potent IL-12 antagonist. Eur. J. Immunol. 1995, 25, 200–206. [Google Scholar] [CrossRef]
- Dasgupta, S.; Bandopadhyay, M.; Pahan, K. Generation of functional blocking monoclonal antibodies against mouse interleukin-12 p40 homodimer and monomer. Hybridoma 2008, 27, 141–151. [Google Scholar] [CrossRef]
- Mondal, S.; Roy, A.; Pahan, K. Functional blocking monoclonal antibodies against IL-12p40 homodimer inhibit adoptive transfer of experimental allergic encephalomyelitis. J. Immunol. 2009, 182, 5013–5023. [Google Scholar] [CrossRef]
- Kundu, M.; Raha, S.; Roy, A.; Pahan, K. Regression of Triple-Negative Breast Cancer in a Patient-Derived Xenograft Mouse Model by Monoclonal Antibodies against IL-12 p40 Monomer. Cells 2022, 11, 259. [Google Scholar] [CrossRef]
- Kundu, M.; Roy, A.; Pahan, K. Selective neutralization of IL-12 p40 monomer induces death in prostate cancer cells via IL-12-IFN-gamma. Proc. Natl. Acad. Sci. USA 2017, 114, 11482–11487. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Pengal, R.A.; Ganesan, L.P.; Wei, G.; Fang, H.; Ostrowski, M.C.; Tridandapani, S. Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol. Immunol. 2006, 43, 1557–1564. [Google Scholar] [CrossRef]
- Kumaran Satyanarayanan, S.; El Kebir, D.; Soboh, S.; Butenko, S.; Sekheri, M.; Saadi, J.; Peled, N.; Assi, S.; Othman, A.; Schif-Zuck, S.; et al. IFN-beta is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat. Commun. 2019, 10, 3471. [Google Scholar] [CrossRef]
- Karimi, Y.; Giles, E.C.; Vahedi, F.; Chew, M.V.; Nham, T.; Loukov, D.; Lee, A.J.; Bowdish, D.M.; Ashkar, A.A. IFN-beta signalling regulates RAW 264.7 macrophage activation, cytokine production, and killing activity. Innate Immun. 2020, 26, 172–182. [Google Scholar] [CrossRef]
- Guo, J.; He, L.; Yuan, P.; Wang, P.; Lu, Y.; Tong, F.; Wang, Y.; Yin, Y.; Tian, J.; Sun, J. ADAM10 overexpression in human non-small cell lung cancer correlates with cell migration and invasion through the activation of the Notch1 signaling pathway. Oncol. Rep. 2012, 28, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Gorry, M.; Sobo-Vujanovic, A.; Lin, Y.; Vujanovic, L.; Gaither-Davis, A.; Moss, M.L.; Miller, M.A.; Griffith, L.G.; Lauffenburger, D.A.; et al. ADAM10 Sheddase Activity is a Potential Lung-Cancer Biomarker. J. Cancer 2018, 9, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M.; Ericsson, A.C. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front. Immunol. 2021, 12, 620510. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, C.; Li, J.; You, S.; Dang, L.; Wu, J.; Hao, Z.; Li, J.; Zhi, Y.; Chen, L.; et al. Proteomic characterization of four subtypes of M2 macrophages derived from human THP-1 cells. J. Zhejiang Univ. Sci. B 2022, 23, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Nakamura, K.; Kobayashi, M.; Herndon, D.N.; Suzuki, F. CCL1 released from M2b macrophages is essentially required for the maintenance of their properties. J. Leukoc. Biol. 2012, 92, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Tsuchimoto, Y.; Ohama, H.; Fukunishi, S.; Tsuda, Y.; Kobayashi, M.; Higuchi, K.; Suzuki, F. Host antitumor resistance improved by the macrophage polarization in a chimera model of patients with HCC. Oncoimmunology 2017, 6, e1299301. [Google Scholar] [CrossRef] [PubMed]
- Guo, B. IL-10 Modulates Th17 Pathogenicity during Autoimmune Diseases. J. Clin. Cell. Immunol. 2016, 7, 400. [Google Scholar] [CrossRef] [PubMed]
- Spach, K.M.; Nashold, F.E.; Dittel, B.N.; Hayes, C.E. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 6030–6037. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sugimoto, C.; Arainga, M.; Alvarez, X.; Didier, E.S.; Kuroda, M.J. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: Implications for understanding lung disease in humans. J. Immunol. 2014, 192, 2821–2829. [Google Scholar] [CrossRef]
- Jana, M.; Dasgupta, S.; Saha, R.N.; Liu, X.; Pahan, K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J. Neurochem. 2003, 86, 519–528. [Google Scholar] [CrossRef]
- Goswami, B.; Rajappa, M.; Mallika, V.; Shukla, D.K.; Kumar, S. TNF-alpha/IL-10 ratio and C-reactive protein as markers of the inflammatory response in CAD-prone North Indian patients with acute myocardial infarction. Clin. Chim. Acta 2009, 408, 14–18. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, S.; Ahmad, M.K.; Singh, R.; Pradhan, A.; Chandra, S.; Kumar, S. TNF-alpha/IL-10 ratio: An independent predictor for coronary artery disease in North Indian population. Diabetes Metab. Syndr. 2018, 12, 221–225. [Google Scholar] [CrossRef]
- Tsurumi, A.; Que, Y.A.; Ryan, C.M.; Tompkins, R.G.; Rahme, L.G. TNF-alpha/IL-10 Ratio Correlates with Burn Severity and May Serve as a Risk Predictor of Increased Susceptibility to Infections. Front. Public Health 2016, 4, 216. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, J.; Su, X.; Yao, X.; Li, H.; Cheng, J.; Mao, J.; Li, X.; Chen, J.; Lin, W. Serum IL-12p40: A novel biomarker for early prediction of minimal change disease relapse following glucocorticoids therapy. Front. Med. 2022, 9, 922193. [Google Scholar] [CrossRef]
- Shawky, H.; El-Shenawy, R.; Helmy, N.M. Circulating macrophage inflammatory protein-1β/IL-12p40 ratio predicts sofosbuvir-based treatment outcome in HCV- genotype 4 patients. Hum. Antibodies 2021, 29, 263–274. [Google Scholar] [CrossRef]
- Stanilov, N.; Miteva, L.; Jovchev, J.; Cirovski, G.; Stanilova, S. The prognostic value of preoperative serum levels of IL-12p40 and IL-23 for survival of patients with colorectal cancer. APMIS 2014, 122, 1223–1229. [Google Scholar] [CrossRef]
- Alexander, A.F.; Kelsey, I.; Forbes, H.; Miller-Jensen, K. Single-cell secretion analysis reveals a dual role for IL-10 in restraining and resolving the TLR4-induced inflammatory response. Cell Rep. 2021, 36, 109728. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Ghaffari, A.A.; Cheng, G. Lipopolysaccharide-mediated IL-10 transcriptional regulation requires sequential induction of type I IFNs and IL-27 in macrophages. J. Immunol. 2010, 185, 6599–6607. [Google Scholar] [CrossRef]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef]
- Iwasaki, N.; Terawaki, S.; Shimizu, K.; Oikawa, D.; Sakamoto, H.; Sunami, K.; Tokunaga, F. Th2 cells and macrophages cooperatively induce allergic inflammation through histamine signaling. PLoS ONE 2021, 16, e0248158. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed]
- Surolia, R.; Li, F.J.; Wang, Z.; Li, H.; Dsouza, K.; Thomas, V.; Mirov, S.; Pérez-Sala, D.; Athar, M.; Thannickal, V.J.; et al. Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight 2019, 4, e123253. [Google Scholar] [CrossRef] [PubMed]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Markovitz, D.M. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 2003, 5, 59–63. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, B.; Pahan, K. IL-12p40 Monomer: A Potential Player in Macrophage Regulation. Immuno 2024, 4, 77-90. https://doi.org/10.3390/immuno4010005
Jeong B, Pahan K. IL-12p40 Monomer: A Potential Player in Macrophage Regulation. Immuno. 2024; 4(1):77-90. https://doi.org/10.3390/immuno4010005
Chicago/Turabian StyleJeong, Brian, and Kalipada Pahan. 2024. "IL-12p40 Monomer: A Potential Player in Macrophage Regulation" Immuno 4, no. 1: 77-90. https://doi.org/10.3390/immuno4010005
APA StyleJeong, B., & Pahan, K. (2024). IL-12p40 Monomer: A Potential Player in Macrophage Regulation. Immuno, 4(1), 77-90. https://doi.org/10.3390/immuno4010005