Fluorinated Derivatives of Digalloyl-Flavan-3-ol Induce Autophagic Cell Death by Forming Granular Aggregates Containing Mitochondria
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Cell Viability Assay
2.3. Cell Viability Measurement Using Cell Death Inhibitors
2.4. Mitochondrial Superoxide (SOX) Assay
2.5. Mitochondrial Membrane Potential Assay with JC-1
2.6. Mitochondrial Morphological Assay with MitoTrackerTM Red
2.7. Western Blots
2.8. Statistical Analysis
3. Results
3.1. Investigation of Cytotoxicity and Cell Death Induction Mechanism
3.2. Effects of Compounds on Mitochondria and Induction of Mitochondrial Dysfunction
3.3. Confirmation of Autophagy Induction Using LC3-II Accumulation as an Index
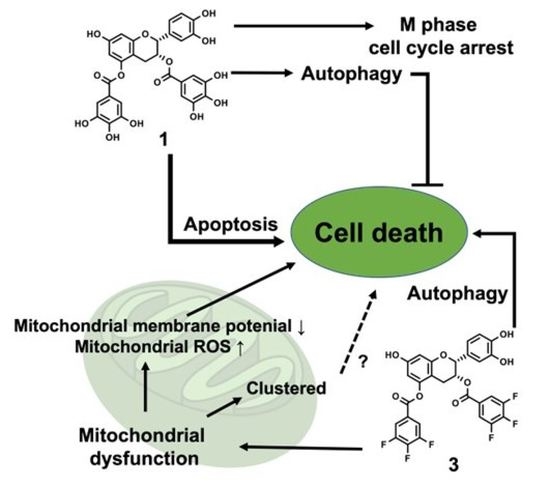
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toda, M.; Okubo, S.; Ikigai, H.; Suzuki, T.; Suzuki, Y.; Hara, Y.; Shimamura, T. The protective activity of tea catechins against experimental infection by Vibrio cholerae O1. Microbiol. Immunol. 1992, 36, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, M. Benifuuki tea suppresses histamine signaling and matrix metalloproteinase—9. Expression in TDI—Sensitized nasal allergy model rats. Jpn. Pharmacol. Ther. 2013, 41, 577–585. [Google Scholar]
- Maeda, Y.M.; Ema, K.; Monobe, M.; Shibuichi, I.; Shinoda, Y.; Yamamoto, T.; Fujisawa, T. The efficacy of early treatment of seasonal allergic rhinitis with benifuuki green tea containing O-methylated catechin before pollen exposure: An open randomized study. Allergol. Int. 2009, 58, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (−)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2013, 61, 875–881. [Google Scholar] [CrossRef]
- Farhan, M. Green tea catechins: Nature’s way of preventing and treating cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic effects of green tea polyphenol (–)-epigallocatechin-3-gallate (EGCG) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int. J. Mol. Sci. 2023, 24, 340. [Google Scholar]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef]
- Kumazoe, M.; Sugihara, K.; Tsukamoto, S.; Huang, Y.; Tsurudome, Y.; Suzuki, T.; Suemasu, Y.; Ueda, N.; Yamashita, S.; Kim, Y.; et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Investig. 2013, 123, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Fujimura, Y.; Hagihara, T.; Sasaki, M.; Yukihira, D.; Nagao, T.; Miura, D.; Yamaguchi, S.; Saito, K.; Tanaka, H.; et al. In situ label-free imaging for visualizing the biotransformation of a bioactive polyphenol. Sci. Rep. 2013, 3, 2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Ayano, Y.; Hamada, Y.; Hojima, T.; Tanaka, R.; Higashino, Y.; Izuno, M.; Okamoto, T.; Kawasaki, T.; Hamada, M.; et al. Role of 2,3-cis structure of (−) -epicatechin-3,5-O-digallate in inhibition of HeLa S3 cell proliferation. Nat. Prod. Chem. Res. 2015, 3, 172. [Google Scholar]
- Hojima, T.; Komeda, S.; Higashino, Y.; Hamada, M.; Nakajima, N.; Kawasaki, T.; Saito, A. Role of 3,5-digalloyl and 3’,4’-dihydroxyl structure of (–)-epicatechin-3,5-digallate in inhibition of Hela S3 cell proliferation. Nat. Prod. Chem. Res. 2017, 5, 250. [Google Scholar] [CrossRef]
- Slee, E.A.; Zhu, H.; Chow, S.C.; MacFarlane, M.; Nicholson, D.W.; Cohen, G.M. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem. J. 1996, 315, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.H.; Kim, Y.T.; Park, S.J. Dieckol inhibits autophagic flux and induces apoptotic cell death in A375 human melanoma cells via lysosomal dysfunction and mitochondrial membrane impairment. Int. J. Mol. Sci. 2022, 23, 14149. [Google Scholar] [CrossRef]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Borges, A.A.; Souza, M.P.; Fonseca, A.C.C.; Wermelinger, G.F.; Ribeiro, R.C.B.; Amaral, A.A.P.; Carvalho, C.J.C.; Abreu, L.S.; Queiroz, L.N.; Almeida, E.C.P.; et al. Chemoselective synthesis of mannich adducts from 1,4-naphthoquinones and profile as autophagic inducers in oral squamous cell carcinoma. Molecules 2023, 28, 309. [Google Scholar]
- Stojanov, S.J.; Kostić, A.; Ljujić, M.; Lupšić, E.; Schenone, S.; Pešić, M.; Dinić, J. Autophagy inhibition enhances anti-glioblastoma effects of pyrazolo[3,4-d]pyrimidine tyrosine kinase inhibitors. Life 2022, 12, 1503. [Google Scholar] [CrossRef] [PubMed]
- Divé, I.; Klann, K.; Michaelis, J.B.; Heinzen, D.; Steinbach, J.P.; Münch, C.; Ronellenfitsch, M.W. Inhibition of mTOR signaling protects human glioma cells from hypoxia-induced cell death in an autophagy-independent manner. Cell Death Discov. 2022, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.S.; Paris, A.; Codron, P.; Cassereau, J.; Procaccio, V.; Lenaers, G.; Reynier, P.; Chevrollier, A. Current mechanistic insights into the CCCP-induced cell survival response. Biochem. Pharmacol. 2018, 148, 100–110. [Google Scholar] [PubMed]
- Panusatid, C.; Thangsiriskul, N.; Peerapittayamongkol, C. Methods for mitochondrial health assessment by high content imaging system. Methods X 2022, 9, 101685. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Prabhu, V.; Mohanty, V.; Krishnaraj, U.; Abdulla, R. Unveiling the arcanum of formalin-fixed paraffin-embedded archival tissue blocks: A valuable resource for genomic DNA extraction. J. Oral Maxillofac. Pathol. 2022, 26, 289. [Google Scholar] [PubMed]
- Kušnierová, P.; Zeman, D.; Hradilic, P.; Čábal, M.; Zapletalová, O. Neurofilament levels in patients with neurological diseases: A comparison of neurofilament light and heavy chain levels. J. Clin. Lab. Anal. 2019, 33, e22948. [Google Scholar] [CrossRef] [Green Version]
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Scheiner, J. Fluorinated pyrimidines. a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef]
- Duschinsky, R.; Pleven, E.; Heidelberger, C. The synthesis of 5-fluoropyrimidines. J. Am. Chem. Soc. 1957, 79, 4559–4560. [Google Scholar] [CrossRef]
- Hoffstrom, B.G.; Kaplan, A.; Letso, R.; Schmid, R.S.; Turmel, G.J.; Lo, D.C.; Stockwell, B.R. Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. Nat. Chem. Biol. 2010, 6, 900–906. [Google Scholar] [CrossRef] [Green Version]
- Daniel, M.K.; Xia, W.; Barbara, A.S.; Omar, A.I.; Marc, R.V.G.; James, E.B.; Michael, C. Ageing and hypoxia cause protein aggregation in mitochondria. Cell Death Diff. 2017, 24, 1730–1738. [Google Scholar]
- Onodera, Y.; Nam, J.M.; Horikawa, M.; Shirato, H.; Sabe, H. Arf6-driven cell invasion is intrinsically linked to TRAK1-mediated mitochondrial anterograde trafficking to avoid oxidative catastrophe. Nat. Commun. 2018, 9, 2682. [Google Scholar] [CrossRef] [Green Version]
- Indelchik, M.D.P.S.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Oowada, S.; Kon, Y.; Hirayama, A.; Yasui, H.; Meike, S.; Kobayashi, S.; Kuwabara, M.; Inanami, O. Redox regulation in radiation-induced cytochrome c release from mitochondria of human lung carcinoma A549 cells. Cancer Lett. 2009, 277, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.C.; Fan, W.; Procaccio, V. Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 2010, 5, 297–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indo, H.P.; Davidson, M.; Yen, H.C.; Suenaga, S.; Tomita, K.; Nishii, T.; Higuchi, M.; Koga, Y.; Ozawa, T.; Majima, H.J. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 2007, 7, 106–118. [Google Scholar] [CrossRef]
- Saita, S.; Shirane, M.; Nakayama, I.K. Selective escape of proteins from the mitochondria during mitophagy. Nat. Commun. 2013, 4, 1410. [Google Scholar] [CrossRef] [Green Version]
- Bock, F.J.; Stephen, W.G.T. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 2, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamammoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, R.S.; Mizushima, N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Higashino, Y.; Okamoto, T.; Mori, K.; Kawasaki, T.; Hamada, M.; Nakajima, N.; Saito, A. Regioselective synthesis of procyanidin B6, a 4-6-condensed (+)-catechin dimer, by intramolecular condensation. Molecules 2018, 23, 205. [Google Scholar] [CrossRef] [Green Version]
- Hamada, Y.; Takano, S.; Ayano, Y.; Tokunaga, M.; Koashi, T.; Okamoto, S.; Doi, S.; Ishida, M.; Kawasaki, T.; Hamada, M.; et al. Structure–activity relationship of oligomeric flavan-3-ols: Importance of the upper-unit B-ring hydroxyl groups in the dimeric structure for strong activities. Molecules 2015, 20, 18870–18885. [Google Scholar] [CrossRef] [Green Version]
- Saito, A. Challenges and complexity of functionality evaluation of flavan-3-ol derivatives. Biosci. Biotechnol. Biochem. 2017, 81, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Saijo, R.; Takeda, Y. HPLC analysis of catechins in various kinds of green teas produced in Japan and abroad. Nippo Shokuhin Kagaku Kogaku Kaishi 1999, 46, 138–147. [Google Scholar] [CrossRef]
- Johnson, B.M.; Shu, Y.Z.; Zhuo, X.; Meanwell, N.A. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Liu, H.; Zhang, R.; Deng, Y.; Hao, Y.; Liao, W.; Yuan, M.; Sun, S. PINK1/Parkin-mediated mitophagy inhibits warangalone-induced mitochondrial apoptosis in breast cancer cells. Aging 2021, 13, 12955–12972. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, S.W.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LoBianco, F.V.; Krager, K.J.; Johnson, E.; Godwin, C.O.; Allen, A.R.; Crooks, P.A.; Compadre, C.M.; Borrelli, M.J.; Aykin-Burns, N. Parthenolide induces rapid thiol oxidation that leads to ferroptosis in hepatocellular carcinoma cells. Font. Toxicol. 2022, 4, 936149. [Google Scholar] [CrossRef]








| Compound | Final Conc. (µM) | HeLa Cells | A549 Cells | ||
|---|---|---|---|---|---|
| Mean ± SD | p-Value (vs. 0 µM) | Mean ± SD | p-Value (vs. 0 µM) | ||
| 1 | 5 | 84.1 ± 5.6 | *** p < 0.001 | 68.4 ± 0.49 | *** p < 0.001 |
| 10 | 63.6 ± 2.8 | *** p < 0.001 | 32.9 ± 2.8 | *** p < 0.001 | |
| 20 | 52.5 ± 1.7 | *** p < 0.001 | 31.1 ± 1.6 | *** p < 0.001 | |
| 30 | 46.8 ± 1.6 | *** p < 0.001 | 28.7 ± 1.2 | *** p < 0.001 | |
| 40 | 46.0 ± 0.98 | *** p < 0.001 | 26.6 ± 1.6 | *** p < 0.001 | |
| 50 | 37.6 ± 2.1 | *** p < 0.001 | 21.3 ± 0.77 | *** p < 0.001 | |
| 60 | 27.9 ± 3.3 | *** p < 0.001 | 16.5 ± 1.2 | *** p < 0.001 | |
| 2 | 5 | 103.1 ± 3.9 | N.S. 1 | 106.8 ± 4.3 | N.S. 1 |
| 10 | 100.2 ± 3.7 | N.S. 1 | 89.1 ± 3.0 | N.S. 1 | |
| 20 | 91.5 ± 7.2 | N.S. 1 | 69.9 ± 3.6 | *** p < 0.001 | |
| 30 | 80.0 ± 2.7 | *** p < 0.001 | 63.9 ± 2.3 | *** p < 0.001 | |
| 40 | 76.9 ± 2.6 | *** p < 0.001 | 57.8 ± 3.0 | *** p < 0.001 | |
| 50 | 71.9 ± 3.5 | *** p < 0.001 | 50.7 ± 2.5 | *** p < 0.001 | |
| 60 | 65.4 ± 3.2 | *** p < 0.001 | 45.1 ± 1.5 | *** p < 0.001 | |
| 3 | 5 | 91.4 ± 8.3 | N.S. 1 | 98.6 ± 3.6 | N.S. 1 |
| 10 | 20.4 ± 2.7 | *** p < 0.001 | 39.1 ± 1.8 | *** p < 0.001 | |
| 20 | 7.7 ± 0.96 | *** p < 0.001 | 12.9 ± 1.4 | *** p < 0.001 | |
| 30 | 3.3 ± 0.11 | *** p < 0.001 | 2.4 ± 0.21 | *** p < 0.001 | |
| 40 | 3.3 ± 0.15 | *** p < 0.001 | 1.6 ± 0.060 | *** p < 0.001 | |
| 50 | 3.3 ± 0.084 | *** p < 0.001 | 1.6 ± 0.041 | *** p < 0.001 | |
| 60 | 3.2 ± 0.13 | *** p < 0.001 | 1.6 ± 0.059 | *** p < 0.001 | |
| 4 | 5 | 93.4 ± 4.8 | N.S. 1 | 100.9 ± 5.0 | N.S. 1 |
| 10 | 39.4 ± 5.3 | *** p < 0.001 | 71.7 ± 4.7 | *** p < 0.001 | |
| 20 | 14.9 ± 0.79 | *** p < 0.001 | 33.5 ± 4.2 | *** p < 0.001 | |
| 30 | 3.8 ± 0.33 | *** p < 0.001 | 7.1 ± 1.0 | *** p < 0.001 | |
| 40 | 3.4 ± 0.14 | *** p < 0.001 | 2.2 ± 0.33 | *** p < 0.001 | |
| 50 | 3.4 ± 0.076 | *** p < 0.001 | 1.7 ± 0.18 | *** p < 0.001 | |
| 60 | 3.4 ± 0.087 | *** p < 0.001 | 1.6 ± 0.073 | *** p < 0.001 | |
| Compound | HeLa cells IC50 µM ± SD | A549 cells IC50 µM ± SD |
|---|---|---|
| 1 | 23.6 ± 2.5 | 7.16 ± 0.14 |
| 2 | >60 | 51.1 ± 3.4 |
| 3 | 7.49 ± 0.19 | 8.81 ± 0.12 |
| 4 | 8.73 ± 1.7 | 14.8 ± 0.60 |
| HeLa Cells | A549 Cells | |||
|---|---|---|---|---|
| Mean ± SD | p-Value 1 (vs. DMSO) | Mean ± SD | p-Value 1 (vs. DMSO) | |
| DMSO + BAF | 6.2 ± 0.20 | *** p < 0.001 | 2.2 ± 0.044 | *** p < 0.001 |
| 1 | 2.0 ± 0.090 | *** p < 0.001 | 3.6 ± 0.069 | *** p < 0.001 |
| 1 + BAF | 6.5 ± 0.18 | *** p < 0.001 | 4.2 ± 0.090 | *** p < 0.001 |
| 2 | 1.4 ± 0.078 | 0.0083 * | 2.9 ± 0.064 | *** p < 0.001 |
| 2 + BAF | 6.7 ± 0.41 | *** p < 0.001 | 5.2 ± 0.14 | *** p < 0.001 |
| 3 | 3.2 ± 0.30 | *** p < 0.001 | 3.4 ± 0.18 | *** p < 0.001 |
| 3 + BAF | 10.9 ± 0.68 | *** p < 0.001 | 3.2 ± 0.16 | *** p < 0.001 |
| 4 | 3.0 ± 0.21 | *** p < 0.001 | 3.0 ± 0.14 | *** p < 0.001 |
| 4 + BAF | 13.4 ± 0.39 | *** p < 0.001 | 2.9 ± 0.088 | *** p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doge, R.; Nishino, Y.; Saito, A. Fluorinated Derivatives of Digalloyl-Flavan-3-ol Induce Autophagic Cell Death by Forming Granular Aggregates Containing Mitochondria. BioChem 2023, 3, 61-77. https://doi.org/10.3390/biochem3020005
Doge R, Nishino Y, Saito A. Fluorinated Derivatives of Digalloyl-Flavan-3-ol Induce Autophagic Cell Death by Forming Granular Aggregates Containing Mitochondria. BioChem. 2023; 3(2):61-77. https://doi.org/10.3390/biochem3020005
Chicago/Turabian StyleDoge, Ryo, Yuki Nishino, and Akiko Saito. 2023. "Fluorinated Derivatives of Digalloyl-Flavan-3-ol Induce Autophagic Cell Death by Forming Granular Aggregates Containing Mitochondria" BioChem 3, no. 2: 61-77. https://doi.org/10.3390/biochem3020005
APA StyleDoge, R., Nishino, Y., & Saito, A. (2023). Fluorinated Derivatives of Digalloyl-Flavan-3-ol Induce Autophagic Cell Death by Forming Granular Aggregates Containing Mitochondria. BioChem, 3(2), 61-77. https://doi.org/10.3390/biochem3020005