Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management
Abstract
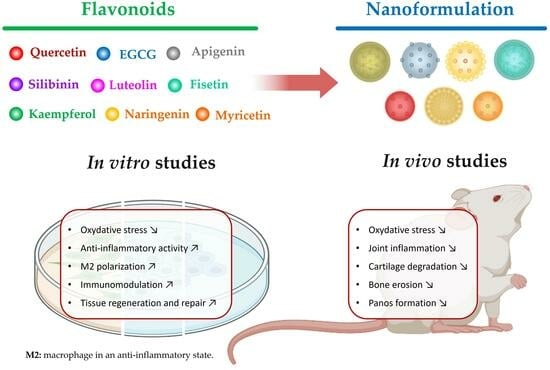
:1. Introduction
2. Flavonoids’ Anti-Arthritic Activities and Clinical Trial Potential
2.1. Quercetin
2.2. Epigallocatechin-3-Gallate (EGCG)
2.3. Apigenin
2.4. Luteolin
2.5. Fisetin
2.6. Silibinin
2.7. Kaempferol
2.8. Naringenin
2.9. Myricetin
3. Challenges in Harnessing Flavonoids for Anti-Inflammatory Applications and the Potential of Nanoparticles
3.1. Bioavailability Limitations
3.2. Nanoparticles as Delivery Vehicles
3.3. Targeted Delivery to Inflammatory Sites
3.4. Prolonged Circulation Time
3.5. Enabling Diverse Compounds’ Encapsulation and Synergy
4. Flavonoid-Based Nanoparticles Enhance the Therapeutic Potential
4.1. Quercetin-Based Nanoparticles
4.2. EGCG-Based Nanoparticles
4.3. Fisetin-Based Nanoparticles
4.4. Naringenin-Based Nanoparticles
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- Stanich, J.A.; Carter, J.D.; Whittum-Hudson, J.; Hudson, A.P. Rheumatoid arthritis: Disease or syndrome? Open Access Rheumatol. Res. Rev. 2009, 1, 179–192. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lu, B.; Guan, H.; Greenberg, J.D.; Kremer, J.; Solomon, D.H. Physician Prescribing Patterns and Risk of Future Long-Term Opioid Use Among Patients With Rheumatoid Arthritis: A Prospective Observational Cohort Study. Arthritis Rheumatol. 2020, 72, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.N.; Zaman Huri, H.; Yahya, F. Drug-related problems in patients with rheumatoid arthritis. Ther. Clin. Risk Manag. 2019, 15, 505–524. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Ndayambaje, M.; Wahnou, H.; Sow, M.; Chgari, O.; Habyarimana, T.; Karkouri, M.; Limami, Y.; Naya, A.; Oudghiri, M. Exploring the multifaceted effects of Ammi visnaga: Subchronic toxicity, antioxidant capacity, immunomodulatory, and anti-inflammatory activities. J. Toxicol. Environ. Health Part A 2024, 87, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Limami, Y.; Pinon, A.; Wahnou, H.; Oudghiri, M.; Liagre, B.; Simon, A.; Duval, R.E. Ursolic Acid’s Alluring Journey: One Triterpenoid vs. Cancer Hallmarks. Molecules 2023, 28, 7897. [Google Scholar] [CrossRef]
- Benayad, S.; Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Oudghiri, M.; Saad, E.M.; Duval, R.E.; Limami, Y. The Promise of Piperine in Cancer Chemoprevention. Cancers 2023, 15, 5488. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; Liagre, B.; Sol, V.; El Attar, H.; Attar, R.; Oudghiri, M.; Duval, R.E.; Limami, Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers 2023, 15, 3826. [Google Scholar] [CrossRef]
- Hunter, D.J.; Guermazi, A.; Roemer, F.; Zhang, Y.; Neogi, T. Structural correlates of pain in joints with osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1170–1178. [Google Scholar] [CrossRef]
- Limami, Y.; Leger, D.Y.; Liagre, B.; Pécout, N.; Viana, M. Ibuprofen-loaded calcium phosphate granules: A new bone substitute for local relieving symptoms of osteoarthritis. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2021, 158, 105679. [Google Scholar] [CrossRef]
- Guo, X.; Lou, J.; Wang, F.; Fan, D.; Qin, Z. Recent Advances in Nano-Therapeutic Strategies for Osteoarthritis. Front. Pharmacol. 2022, 13, 924387. [Google Scholar] [CrossRef]
- Wahnou, H.; Youlyouz-Marfak, I.; Liagre, B.; Sol, V.; Oudghiri, M.; Duval, R.E.; Limami, Y. Shining a Light on Prostate Cancer: Photodynamic Therapy and Combination Approaches. Pharmaceutics 2023, 15, 1767. [Google Scholar] [CrossRef] [PubMed]
- Hba, S.; Ghaddar, S.; Wahnou, H.; Pinon, A.; El Kebbaj, R.; Pouget, C.; Sol, V.; Liagre, B.; Oudghiri, M.; Limami, Y. Natural Chalcones and Derivatives in Colon Cancer: Pre-Clinical Challenges and the Promise of Chalcone-Based Nanoparticles. Pharmaceutics 2023, 15, 2718. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Internet J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Rudrapal, M.; Eltayeb, W.A.; Rakshit, G.; El-Arabey, A.A.; Khan, J.; Aldosari, S.M.; Alshehri, B.; Abdalla, M. Dual synergistic inhibition of COX and LOX by potential chemicals from Indian daily spices investigated through detailed computational studies. Sci. Rep. 2023, 13, 8656. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.N.; Han, C.; Song, P.Y.; Zhao, X.H.; Zhong, X.H. Anti-inflammatory effects of luteolin and quercetin in vitro. Prog. Veter. Med. 2017, 38, 56–61. [Google Scholar]
- Cessak, G.; Kuzawińska, O.; Burda, A.; Lis, K.; Wojnar, M.; Mirowska-Guzel, D.; Bałkowiec-Iskra, E. TNF inhibitors—Mechanisms of action, approved and off-label indications. Pharmacol. Rep. PR 2014, 66, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.T.; Gohil, V.M.; Bhutani, K.K. Modulating TNF-alpha signaling with natural products. Drug Discov. Today 2006, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.Y.; Zhang, B.Y.; Huang, J.L. Protective effects of quercetin on the inflammation of mice RAW264. 7 cells induced by LPS. Chin. Traditional. Patent Med. 2019, 8, 1795–1799. [Google Scholar]
- Pereira, G.S.; Percebom, I.; Mendes, S.; Souza, P.S.S.; Diniz, L.F.A.; Costa, M.F.; Lopes, B.R.P.; Toledo, K.A. Quercetin inhibits neutrophil extracellular traps release and their cytotoxic effects on A549 cells, as well the release and enzymatic activity of elastase and myeloperoxidase. Braz. J. Biol. 2022, 84, e252936. [Google Scholar] [CrossRef]
- Song, W.; Ye, J.; Pan, N.; Tan, C.; Herrmann, M. Neutrophil Extracellular Traps Tied to Rheumatoid Arthritis: Points to Ponder. Front. Immunol. 2021, 11, 578129. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Wang, H.-M.; Chen, P.-Y.; Wu, T.-C. Interactions of β-carotene and flavonoids on the secretion of pro-inflammatory mediators in an in vitro system. Chem.-Biol. Interact. 2009, 179, 386–393. [Google Scholar] [CrossRef]
- Avila, C.M.; Romeiro, N.C.; Sant’Anna, C.M.; Barreiro, E.J.; Fraga, C.A. Structural insights into IKKbeta inhibition by natural products staurosporine and quercetin. Bioorganic Med. Chem. Lett. 2009, 19, 6907–6910. [Google Scholar] [CrossRef]
- Musumeci, D.; Roviello, G.N.; Montesarchio, D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol. Ther. 2014, 141, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kawahara, K.; Yone, K.; Hashiguchi, T.; Yamakuchi, M.; Goto, M.; Inoue, K.; Yamada, S.; Ijiri, K.; Matsunaga, S.; et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003, 48, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Gardi, C.; Bauerova, K.; Stringa, B.; Kuncirova, V.; Slovak, L.; Ponist, S.; Drafi, F.; Bezakova, L.; Tedesco, I.; Acquaviva, A.; et al. Quercetin reduced inflammation and increased antioxidant defense in rat adjuvant arthritis. Arch. Biochem. Biophys. 2015, 583, 150–157. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Miranda-Hernandez, S.; Alim, M.A.; Hayes, L.; Bird, G.; Ketheesan, N. Therapeutic effect of quercetin in collagen-induced arthritis. Biomed. Pharmacother. 2017, 90, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Xu, M.; Wu, X.; Zhao, F.; Zhao, C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 2018, 54, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Chen, L.U.; Zhang, C.; Fujita, M.; Li, R.; Yan, S.-M.; Ong, C.K.; Liao, X.; Gao, Q.; Sasagawa, S. Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell 2019, 35, 932–947. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Staurengo-Ferrari, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Ruiz-Miyazawa, K.W.; Vicentini, F.; Vignoli, J.A.; Camilios-Neto, D.; Georgetti, S.R.; Baracat, M.M.; et al. Quercetin attenuates zymosan-induced arthritis in mice. Biomed. Pharmacother. 2018, 102, 175–184. [Google Scholar] [CrossRef]
- Guan, F.; Wang, Q.; Bao, Y.; Chao, Y. Anti-rheumatic effect of quercetin and recent developments in nano formulation. RSC Adv. 2021, 11, 7280–7293. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, H.; Liu, C.; Huang, J.; Liu, Z. A review for physiological activities of EGCG and the role in improving fertility in humans/mammals. Biomed. Pharmacother. 2020, 127, 110186. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Ahmed, S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: Progress and promise. Arthritis Res. Ther. 2010, 12, 208. [Google Scholar] [CrossRef]
- Ahmed, S.; Rahman, A.; Hasnain, A.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic. Biol. Med. 2002, 33, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahmed, S.; Malemud, C.J.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2003, 21, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Adcocks, C.; Collin, P.; Buttle, D.J. Catechins from green tea (Camellia sinensis) inhibit bovine and human cartilage proteoglycan and type II collagen degradation in vitro. J. Nutr. 2002, 132, 341–346. [Google Scholar] [CrossRef]
- Vankemmelbeke, M.N.; Jones, G.C.; Fowles, C.; Ilic, M.Z.; Handley, C.J.; Day, A.J.; Knight, C.G.; Mort, J.S.; Buttle, D.J. Selective inhibition of ADAMTS-1, -4 and -5 by catechin gallate esters. Eur. J. Biochem. 2003, 270, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Ahmed, S.; Wang, N.; Gupta, S.; Zhang, A.; Haqqi, T.M. Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with downregulation of nuclear factor-kappaB. Toxicol. Appl. Pharmacol. 2006, 216, 11–19. [Google Scholar] [CrossRef]
- Lee, J.H.; Jin, H.; Shim, H.E.; Kim, H.N.; Ha, H.; Lee, Z.H. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol. Pharmacol. 2010, 77, 17–25. [Google Scholar] [CrossRef]
- Kamon, M.; Zhao, R.; Sakamoto, K. Green tea polyphenol (-)-epigallocatechin gallate suppressed the differentiation of murine osteoblastic MC3T3-E1 cells. Cell Biol. Int. 2009, 34, 109–116. [Google Scholar] [CrossRef]
- Tokuda, H.; Takai, S.; Hanai, Y.; Matsushima-Nishiwaki, R.; Hosoi, T.; Harada, A.; Ohta, T.; Kozawa, O. (-)-Epigallocatechin gallate suppresses endothelin-1-induced interleukin-6 synthesis in osteoblasts: Inhibition of p44/p42 MAP kinase activation. FEBS Lett. 2007, 581, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Pakozdi, A.; Koch, A.E. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2006, 54, 2393–2401. [Google Scholar] [CrossRef]
- Cronstein, B.N. Interleukin-6--a key mediator of systemic and local symptoms in rheumatoid arthritis. Bull. NYU Hosp. Jt. Dis. 2007, 65 (Suppl. S1), S11–S15. [Google Scholar]
- Ahmed, S.; Marotte, H.; Kwan, K.; Ruth, J.H.; Campbell, P.L.; Rabquer, B.J.; Pakozdi, A.; Koch, A.E. Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production. Proc. Natl. Acad. Sci. USA 2008, 105, 14692–14697. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Silverman, M.D.; Marotte, H.; Kwan, K.; Matuszczak, N.; Koch, A.E. Down-regulation of myeloid cell leukemia 1 by epigallocatechin-3-gallate sensitizes rheumatoid arthritis synovial fibroblasts to tumor necrosis factor alpha-induced apoptosis. Arthritis Rheum. 2009, 60, 1282–1293. [Google Scholar] [CrossRef]
- Liu, H.; Eksarko, P.; Temkin, V.; Haines, G.K., 3rd; Perlman, H.; Koch, A.E.; Thimmapaya, B.; Pope, R.M. Mcl-1 is essential for the survival of synovial fibroblasts in rheumatoid arthritis. J. Immunol. 2005, 175, 8337–8345. [Google Scholar] [CrossRef]
- Haqqi, T.M.; Anthony, D.D.; Gupta, S.; Ahmad, N.; Lee, M.S.; Kumar, G.K.; Mukhtar, H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc. Natl. Acad. Sci. USA 1999, 96, 4524–4529. [Google Scholar] [CrossRef]
- Kim, H.R.; Rajaiah, R.; Wu, Q.L.; Satpute, S.R.; Tan, M.T.; Simon, J.E.; Berman, B.M.; Moudgil, K.D. Green tea protects rats against autoimmune arthritis by modulating disease-related immune events. J. Nutr. 2008, 138, 2111–2116. [Google Scholar] [CrossRef]
- Marotte, H.; Ruth, J.H.; Campbell, P.L.; Koch, A.E.; Ahmed, S. Green tea extract inhibits chemokine production, but up-regulates chemokine receptor expression, in rheumatoid arthritis synovial fibroblasts and rat adjuvant-induced arthritis. Rheumatology 2010, 49, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Tomou, E.M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules 2023, 28, 2387. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.N.; Huang, T.F.; Teng, C.M. Vasodilatory action mechanisms of apigenin isolated from Apium graveolens in rat thoracic aorta. Biochim. Biophys. Acta 1991, 1115, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Sadeer, N.B.; Hussain, M.; Mahwish; Alsagaby, S.A.; Imran, M.; Mumtaz, T.; Umar, M.; Tauseef, A.; Al Abdulmonem, W.; et al. Therapeutical properties of apigenin: A review on the experimental evidence and basic mechanisms. Int. J. Food Prop. 2023, 26, 1914–1939. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Izzi, V.; Masuelli, L.; Tresoldi, I.; Sacchetti, P.; Modesti, A.; Galvano, F.; Bei, R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front. Biosci.-Landmark 2012, 17, 2396–2418. [Google Scholar] [CrossRef]
- Kim, S.; Joo, Y.E. Theaflavin Inhibits LPS-Induced IL-6, MCP-1, and ICAM-1 Expression in Bone Marrow-Derived Macrophages Through the Blockade of NF-κB and MAPK Signaling Pathways. Chonnam Med. J. 2011, 47, 104–110. [Google Scholar] [CrossRef]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef]
- Jeong, G.S.; Lee, S.H.; Jeong, S.N.; Kim, Y.C.; Kim, E.C. Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. Int. Immunopharmacol. 2009, 9, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed]
- Shoara, R.; Hashempur, M.H.; Ashraf, A.; Salehi, A.; Dehshahri, S.; Habibagahi, Z. Efficacy and safety of topical Matricaria chamomilla L. (chamomile) oil for knee osteoarthritis: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2015, 21, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M. In vitro study of molecular structure and cytotoxicity effect of luteolin in the human colon carcinoma cells. Eur. Food Res. Technol. 2015, 241, 83–90. [Google Scholar] [CrossRef]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol. Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, T.; Ren, Y.; Rao, H.; Lei, J.; Zhao, G.; Wang, M.; Gong, D.; Cao, Z. A Literature Review on the Antiviral Mechanism of Luteolin. Nat. Prod. Commun. 2023, 18, 1934578X231171521. [Google Scholar] [CrossRef]
- Lee, J.-P.; Li, Y.-C.; Chen, H.-Y.; Lin, R.-H.; Huang, S.-S.; Chen, H.-L.; Kuan, P.-C.; Liao, M.-F.; Chen, C.-J.; Kuan, Y.-H. Protective effects of luteolin against lipopolysaccharide-induced acute lung injury involves inhibition of MEK/ERK and PI3K/Akt pathways in neutrophils. Acta Pharmacol. Sin. 2010, 31, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, S.Y.; Kim, Y.S.; Lee, W.-H.; Hwang, D.H.; Lee, J.Y. Suppression of the TRIF-dependent signaling pathway of Toll-like receptors by luteolin. Biochem. Pharmacol. 2009, 77, 1391–1400. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, M.S.; Lee, D.H.; Kwon, T.H.; Song, H.-H.; Oh, S.-R.; Yoon, D.Y. Luteolin 8-C-β-fucopyranoside downregulates IL-6 expression by inhibiting MAPKs and the NF-κB signaling pathway in human monocytic cells. Pharmacol. Rep. 2015, 67, 581–587. [Google Scholar] [CrossRef]
- Kang, B.J.; Ryu, J.; Lee, C.J.; Hwang, S.C. Luteolin Inhibits the Activity, Secretion and Gene Expression of MMP-3 in Cultured Articular Chondrocytes and Production of MMP-3 in the Rat Knee. Biomol. Ther. 2014, 22, 239–245. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Pearlman, R.L.; Afaq, F. Fisetin and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 213–244. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Demchuk, O.M. New Perspectives for Fisetin. Front. Chem. 2019, 7, 697. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, H.; Zhang, H.; Feng, X.; Yang, L.; Hou, D.X.; Chen, J. Fisetin inhibits inflammation and induces autophagy by mediating PI3K/AKT/mTOR signaling in LPS-induced RAW264.7 cells. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Molagoda, I.M.N.; Jayasingha, J.A.C.C.; Choi, Y.H.; Jayasooriya, R.G.P.T.; Kang, C.-H.; Kim, G.-Y. Fisetin inhibits lipopolysaccharide-induced inflammatory response by activating β-catenin, leading to a decrease in endotoxic shock. Sci. Rep. 2021, 11, 8377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef]
- Kostek, H.; Szponar, J.; Tchórz, M.; Majewska, M.; Lewandowska-Stanek, H. Silibinin and its hepatoprotective action from the perspective of a toxicologist. Prz. Lek. 2012, 69, 541–543. [Google Scholar]
- Tong, W.W.; Zhang, C.; Hong, T.; Liu, D.H.; Wang, C.; Li, J.; He, X.K.; Xu, W.D. Silibinin alleviates inflammation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes and has a therapeutic effect on arthritis in rats. Sci. Rep. 2018, 8, 3241. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, S.-L.; Mai, C.-T.; Zheng, Y.-F.; Wang, H.; Liu, Z.-Q.; Zhou, H.; Liu, L. Suppression of up-regulated LXRα by silybin ameliorates experimental rheumatoid arthritis and abnormal lipid metabolism. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 80, 153339. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhao, H.; Xu, H.T.; Han, X.D.; Wu, Y.S.; Xu, F.F.; Yang, X.B.; Göransson, U.; Liu, B. Kaempferia galanga L.: Progresses in Phytochemistry, Pharmacology, Toxicology and Ethnomedicinal Uses. Front. Pharmacol. 2021, 12, 675350. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol Alleviates the Interleukin-1β-Induced Inflammation in Rat Osteoarthritis Chondrocytes via Suppression of NF-κB. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3925–3931. [Google Scholar] [CrossRef] [PubMed]
- Aa, L.-X.; Fei, F.; Qi, Q.; Sun, R.-B.; Gu, S.-H.; Di, Z.-Z.; Aa, J.-Y.; Wang, G.-J.; Liu, C.-X. Rebalancing of the gut flora and microbial metabolism is responsible for the anti-arthritis effect of kaempferol. Acta Pharmacol. Sin. 2020, 41, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, C.; Xu, W.; Li, F.; Xu, C.; Wu, C.; Wang, Y.; Zhang, X.; Xia, D. Kaempferol suppresses inflammation in mice suffering from both hyperuricemia and gouty arthritis through inhibiting NLRP3 inflammasome and NF-κB pathway. Res. Square 2023. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, A.; Monga, V.; Bhatia, R. Compendium of naringenin: Potential sources, analytical aspects, chemistry, nutraceutical potentials and pharmacological profile. Crit. Rev. Food Sci. Nutr. 2023, 63, 8868–8899. [Google Scholar] [CrossRef] [PubMed]
- Uçar, K.; Göktaş, Z. Biological activities of naringenin: A narrative review based on in vitro and in vivo studies. Nutr. Res. 2023, 119, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Manchope, M.F.; Casagrande, R.; Verri, W.A., Jr. Naringenin: An analgesic and anti-inflammatory citrus flavanone. Oncotarget 2017, 8, 3766–3767. [Google Scholar] [CrossRef]
- Wang, C.C.; Guo, L.; Tian, F.D.; An, N.; Luo, L.; Hao, R.H.; Wang, B.; Zhou, Z.H. Naringenin regulates production of matrix metalloproteinases in the knee-joint and primary cultured articular chondrocytes and alleviates pain in rat osteoarthritis model. Braz. J. Med. Biol. Res. 2017, 50, e5714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Zhang, J.; Deng, C.; Zhang, C. Naringenin ameliorates collagen-induced arthritis through activating AMPK-mediated autophagy in macrophages. Immun. Inflamm. Dis. 2023, 11, e983. [Google Scholar] [CrossRef] [PubMed]
- Aihaiti, Y.; Cai, Y.S.; Tuerhong, X.; Yang, Y.N.; Ma, Y.; Zheng, H.S.; Xu, K.; Xu, P. Therapeutic Effects of Naringin in Rheumatoid Arthritis: Network Pharmacology and Experimental Validation. Front. Pharmacol. 2021, 12, 672054. [Google Scholar] [CrossRef]
- Ozcan, C.; Yaman, M. Determination of Myricetin in medicinal plants by high-performance liquid chromatography. Instrum. Sci. Technol. 2015, 43, 44–52. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, Y.; Hua, X.; Deng, X.; Sun, P.; Yu, C.; Chen, L.; Yu, S.; Liu, S.; Pang, H. Myricetin ameliorates the symptoms of collagen-induced arthritis in mice by inhibiting cathepsin K activity. Immunopharmacol. Immunotoxicol. 2015, 37, 513–519. [Google Scholar] [CrossRef]
- Jose, A.M.; Rasool, M. Myricetin ameliorates the IL-21-induced tumorigenic phenotype of adjuvant-induced arthritis FLS by modulating the choline kinase signaling cascade. In Vitro Cell. Dev. Biol.—Anim. 2023, 59, 811–820. [Google Scholar] [CrossRef]
- Maus, A.; Strait, L.; Zhu, D. Nanoparticles as delivery vehicles for antiviral therapeutic drugs. Eng. Regen. 2021, 2, 31–46. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Cerqueira, S.R.; Ayad, N.G.; Lee, J.K. Neuroinflammation Treatment via Targeted Delivery of Nanoparticles. Front. Cell. Neurosci. 2020, 14, 576037. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The interaction between nanoparticles and immune system: Application in the treatment of inflammatory diseases. J. Nanobiotechnol. 2022, 20, 127. [Google Scholar] [CrossRef]
- Brusini, R.; Varna, M.; Couvreur, P. Advanced nanomedicines for the treatment of inflammatory diseases. Adv. Drug Deliv. Rev. 2020, 157, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, Y.; Sun, Q.; Zhou, C.; Hu, S.; Lenahan, C.; Xu, W.; Deng, Y.; Li, G.; Tao, S. Update on Nanoparticle-Based Drug Delivery System for Anti-inflammatory Treatment. Front. Bioeng. Biotechnol. 2021, 9, 630352. [Google Scholar] [CrossRef]
- Yoo, J.W.; Chambers, E.; Mitragotri, S. Factors that control the circulation time of nanoparticles in blood: Challenges, solutions and future prospects. Curr. Pharm. Des. 2010, 16, 2298–2307. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Peng, H.; Yu, Z.; Wang, L.; He, H.; Ma, Y.; Qi, J.; Lu, Y.; Wu, W. The long-circulating effect of pegylated nanoparticles revisited via simultaneous monitoring of both the drug payloads and nanocarriers. Acta Pharm. Sin. B 2022, 12, 2479–2493. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.-H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Shen, W.; Wang, R.; Fan, Q.; Li, Y.; Cheng, Y. Natural polyphenol assisted delivery of single-strand oligonucleotides by cationic polymers. Gene Ther. 2020, 27, 383–391. [Google Scholar] [CrossRef]
- Vaz, G.R.; Carrasco, M.C.F.; Batista, M.M.; Barros, P.A.B.; Oliveira, M.D.C.; Muccillo-Baisch, A.L.; Yurgel, V.C.; Buttini, F.; Soares, F.A.A.; Cordeiro, L.M.; et al. Curcumin and Quercetin-Loaded Lipid Nanocarriers: Development of Omega-3 Mucoadhesive Nanoemulsions for Intranasal Administration. Nanomaterials 2022, 12, 1073. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Tang, X.; Ye, S.; Shao, J.; Tu, L.; Pan, J.; Chen, L.; Liang, G.; Yin, L. Synergistic anti-oxidant and anti-inflammatory effects of ceria/resatorvid co-decorated nanoparticles for acute lung injury therapy. J. Nanobiotechnol. 2023, 21, 502. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Lu, X.; Tian, J. Nanoparticle drug delivery systems for synergistic delivery of tumor therapy. Front. Pharmacol. 2023, 14, 1111991. [Google Scholar] [CrossRef]
- Heeba, G.H.; Mahmoud, M.E.; Hanafy, A.A.E. Anti-inflammatory potential of curcumin and quercetin in rats: Role of oxidative stress, heme oxygenase-1 and TNF-α. Toxicol. Ind. Health 2012, 30, 551–560. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Li, F.; Jiang, Y.; Guan, H.; Wang, D.; Sun-Waterhouse, D.; Wu, M.; Li, D. The synergistic protection of EGCG and quercetin against streptozotocin (STZ)-induced NIT-1 pancreatic β cell damage via upregulation of BCL-2 expression by miR-16-5p. J. Nutr. Biochem. 2021, 96, 108748. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.J. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.; Akhtar, B.; Sharif, A.; Anjum, F.; Pasha, I.; Khan, A.; Akhtar, M.F.; Saleem, A. Quercetin-loaded chitosan nanoparticles ameliorate adjuvant-induced arthritis in rats by regulating anti-oxidant enzymes and downregulating pro- and inflammatory cytokines. Inflammopharmacology 2023, 31, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Qin, J.; Huang, Q.; Jin, Z.; Zheng, L.; Zhao, J.; Qin, Z. Epigallocatechin-3-gallate (EGCG) based metal-polyphenol nanoformulations alleviates chondrocytes inflammation by modulating synovial macrophages polarization. Biomed. Pharmacother. 2023, 161, 114366. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xiao, L.; Yu, C.; Jin, P.; Qin, D.; Xu, Y.; Yin, J.; Liu, Z.; Du, Q. Enhanced Antiarthritic Efficacy by Nanoparticles of (−)-Epigallocatechin Gallate–Glucosamine–Casein. J. Agric. Food Chem. 2019, 67, 6476–6486. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.; Muhammad, F.; Zaheer, Y.; Ali, M.A.; Iqbal, M.; Rehman, M.; Munir, M.U.; Akhtar, B.; Webster, T.J.; Sharif, A.; et al. Synthesis of naringenin loaded lipid based nanocarriers and their in-vivo therapeutic potential in a rheumatoid arthritis model. J. Drug Deliv. Sci. Technol. 2021, 66, 102854. [Google Scholar] [CrossRef]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahnou, H.; Limami, Y.; Oudghiri, M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem 2024, 4, 38-61. https://doi.org/10.3390/biochem4010003
Wahnou H, Limami Y, Oudghiri M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem. 2024; 4(1):38-61. https://doi.org/10.3390/biochem4010003
Chicago/Turabian StyleWahnou, Hicham, Youness Limami, and Mounia Oudghiri. 2024. "Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management" BioChem 4, no. 1: 38-61. https://doi.org/10.3390/biochem4010003
APA StyleWahnou, H., Limami, Y., & Oudghiri, M. (2024). Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem, 4(1), 38-61. https://doi.org/10.3390/biochem4010003