Performance of Stainless-Steel Bipolar Plates (SS-BPPs) in Polymer Electrolyte Membrane Water Electrolyser (PEMWE): A Comprehensive Review
Abstract
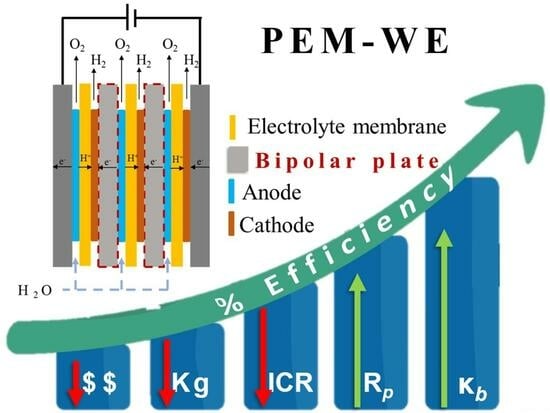
:1. Introduction
2. Metallic BPPs in PEMWE Devices
3. Large Scale, Pilot Scale and Industrial Scale PEMWE Systems
3.1. Large Scale PEMWE Systems
3.2. Pilot Scale PEMWE Systems
3.3. Industrial Scale PEMWE Systems
4. Corrosion Evaluation of Metallic Components in PEMWE Devices
5. Materials and Equipment in PEMWE Systems
6. Ex Situ Testing: Procedures and Methods
7. Discussion
8. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D.A. Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Barbir, F. PEM Electrolysis for Production of Hydrogen from Renewable Energy Sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen Futures: Toward a Sustainable Energy System. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, W.W.; Wang, C.; Mao, Z.Q. Corrosion Behavior of Three Bipolar Plate Materials in Simulated SPE Water Electrolysis Environment. Int. J. Hydrogen Energy 2012, 37, 12069–12073. [Google Scholar] [CrossRef]
- Millet, P.; Andolfatto, F.; Durand, R. Design and performance of a solid polymer electrolyte water electrolyzer. Int. J. Hydrogen Energy 1996, 21, 87–93. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Millet, P.; Volobuev, S.A.; Fateev, V.N. Optimization of Porous Current Collectors for PEM Water Electrolysers. Int. J. Hydrogen Energy 2009, 34, 4968–4973. [Google Scholar] [CrossRef]
- Abd El Meguid, E.A.; Abd El Latif, A.A. Electrochemical and SEM Study on Type 254 SMO Stainless Steel in Chloride Solutions. Corros. Sci. 2004, 46, 2431–2444. [Google Scholar] [CrossRef]
- Wang, P.; Wilson, L.L.; Wesolowski, D.J.; Rosenqvist, J.; Anderko, A. Solution Chemistry of Mo(III) and Mo(IV): Thermodynamic Foundation for Modeling Localized Corrosion. Corros. Sci. 2010, 52, 1625–1634. [Google Scholar] [CrossRef]
- Freitas, M.B.J.G.; Eiras, C.; Bulhoes, L.O.S. Breakdown of the Niobium Oxide Film under Galvanostatic Polarisation and in Acid Solutions. Corros. Sci. 2004, 46, 1051–1060. [Google Scholar] [CrossRef]
- Asselin, E.; Ahmed, T.M.; Alfantazi, A. Corrosion of Niobium in Sulphuric and Hydrochloric Acid Solutions at 75 and 95 °C. Corros. Sci. 2007, 49, 694–710. [Google Scholar] [CrossRef]
- Development of Water Electrolysis in the European Union. Final Report, Fuel Cells and Hydrogen Joint Undertaking, E4tech Sàrl with Element Energy Ltd for the Fuel Cells and Hydrogen Joint Undertaking. 2014. Available online: https://refman.energytransitionmodel.com/publications/2020 (accessed on 21 March 2024).
- Kahraman, H.; Orhan, M.F. Flow Field Bipolar Plates in a Proton Exchange Membrane Fuel Cell: Analysis & Modeling. Energy Convers Manag. 2017, 133, 363–384. [Google Scholar] [CrossRef]
- Barranco, J.; Barreras, F.; Lozano, A.; Lopez, A.M.; Roda, V.; Martin, J.; Maza, M.; Fuentes, G.G.; Almandoz, E. Cr and Zr/Cr Nitride CAE-PVD Coated Aluminum Bipolar Plates for Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2010, 35, 11489–11498. [Google Scholar] [CrossRef]
- Lædre, S.; Kongstein, O.E.; Oedegaard, A.; Karoliussen, H.; Seland, F. Materials for Proton Exchange Membrane Water Electrolyzer Bipolar Plates. Int. J. Hydrogen Energy 2017, 42, 2713–2723. [Google Scholar] [CrossRef]
- Langemann, M.; Fritz, D.L.; Müller, M.; Stolten, D. Validation and Characterization of Suitable Materials for Bipolar Plates in PEM Water Electrolysis. Int. J. Hydrogen Energy 2015, 40, 11385–11391. [Google Scholar] [CrossRef]
- Goetz, M. Metallic Bipolar Plates for PEM Fuel Cells—Status, Potential and Challenges; ElringKlinger AG: Dettingen an der Erms, Germany. Available online: https://docplayer.net/47499358-Metallic-bipolar-plates-for-pem-fuel-cells-status-potential-and-challenges-michael-goetz-elringklinger-ag.html (accessed on 21 March 2024).
- Gautam, R.K.; Banerjee, S.; Kar, K.K. Bipolar Plate Materials for Proton Exchange Membrane Fuel Cell Application. Recent Pat. Mater. Sci. 2015, 8, 15–45. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, M.; Lin, G.; Han, Z.; Fu, Y.; Sun, S.; Shao, Z.; Yi, B. Performance of Ti-Ag-Deposited Titanium Bipolar Plates in Simulated Unitized Regenerative Fuel Cell (URFC) Environment. Int. J. Hydrogen Energy 2011, 36, 5695–5701. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, L.; Lin, G.; Shao, Z. Honeycomb-like Nanocomposite Ti-Ag-N Films Prepared by Pulsed Bias Arc Ion Plating on Titanium as Bipolar Plates for Unitized Regenerative Fuel Cells. J. Power Sources 2012, 198, 196–202. [Google Scholar] [CrossRef]
- Jung, H.Y.; Huang, S.Y.; Ganesan, P.; Popov, B.N. Performance of Gold-Coated Titanium Bipolar Plates in Unitized Regenerative Fuel Cell Operation. J. Power Sources 2009, 194, 972–975. [Google Scholar] [CrossRef]
- Jung, H.Y.; Huang, S.Y.; Popov, B.N. High-Durability Titanium Bipolar Plate Modified by Electrochemical Deposition of Platinum for Unitized Regenerative Fuel Cell (URFC). J. Power Sources 2010, 195, 1950–1956. [Google Scholar] [CrossRef]
- Lin, M.T.; Wan, C.H.; Wu, W. Comparison of Corrosion Behaviors between SS304 and Ti Substrate Coated with (Ti,Zr)N Thin Films as Metal Bipolar Plate for Unitized Regenerative Fuel Cell. Thin Solid Film. 2013, 544, 162–169. [Google Scholar] [CrossRef]
- Toops, T.J.; Brady, M.P.; Zhang, F.Y.; Meyer, H.M.; Ayers, K.; Roemer, A.; Dalton, L. Evaluation of Nitrided Titanium Separator Plates for Proton Exchange Membrane Electrolyzer Cells. J. Power Sources 2014, 272, 954–960. [Google Scholar] [CrossRef]
- Nikiforov, A.V.; Petrushina, I.M.; Christensen, E.; Tomás-García, A.L.; Bjerrum, N.J. Corrosion Behaviour of Construction Materials for High Temperature Steam Electrolysers. Int. J. Hydrogen Energy 2011, 36, 111–119. [Google Scholar] [CrossRef]
- Chisholm, G.; Kitson, P.J.; Kirkaldy, N.D.; Bloor, L.G.; Cronin, L. 3D Printed Flow Plates for the Electrolysis of Water: An Economic and Adaptable Approach to Device Manufacture. Energy Environ. Sci. 2014, 7, 3026–3032. [Google Scholar] [CrossRef]
- Kim, K.M.; Park, J.H.; Kim, J.H.; Kim, K.Y. Effect of Chemical and Heat Treatment on the Interfacial Contact Resistance and Corrosion Resistance of 446M Ferritic Stainless Steel as a Bipolar Plate for Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2011, 36, 9926–9935. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, J.H.; Lee, Y.Y.; Kim, K.Y. Electrodeposition of Ruthenium Oxide on Ferritic Stainless Steel Bipolar Plate for Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2012, 37, 1653–1660. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, J.H.; Lee, Y.Y.; Kim, K.Y. Effect of Immersion in NaOH Solution on Ferritic Stainless Steel as a Bipolar Plate for Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2011, 36, 13014–13021. [Google Scholar] [CrossRef]
- PEM Electrolyzer Incorporating an Advanced Low-Cost Membrane. Available online: https://www.osti.gov/biblio/1091385 (accessed on 21 March 2024).
- Djukic, M.B.; Sijacki Zeravcic, V.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen Damage of Steels: A Case Study and Hydrogen Embrittlement Model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Gago, A.S.; Ansar, S.A.; Saruhan, B.; Schulz, U.; Lettenmeier, P.; Cañas, N.A.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Arnold, J.; et al. Protective Coatings on Stainless Steel Bipolar Plates for Proton Exchange Membrane (PEM) Electrolysers. J. Power Sources 2016, 307, 815–825. [Google Scholar] [CrossRef]
- Gago, A.S.; Ansar, A.S.; Gazdzicki, P.; Wagner, N.; Arnold, J.; Friedrich, K.A. Low Cost Bipolar Plates for Large Scale PEM Electrolyzers. ECS Trans. 2014, 64, 1039–1048. [Google Scholar] [CrossRef]
- Hansen, R.S.; Johnsen, S.E.; Fell, H.J.; Rasten, E. Use of Austenitic Stainless Steel as Construction Material in a Device or Structural Component Which Is Exposed to an Oxygen and/or Hydrogen and/or Hydrofluoric Acid Environment. US20100133096A1, 3 June 2010. [Google Scholar]
- Feng, K.; Li, Z.; Cai, X.; Chu, P.K. Corrosion Behavior and Electrical Conductivity of Niobium Implanted 316L Stainless Steel Used as Bipolar Plates in Polymer Electrolyte Membrane Fuel Cells. Surf. Coat. Technol. 2010, 205, 85–91. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Saruhan, B.; Freitag, O.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Gago, A.S.; Friedrich, K.A. Low-Cost and Durable Bipolar Plates for Proton Exchange Membrane Electrolyzers. Sci. Rep. 2017, 7, 44035–44046. [Google Scholar] [CrossRef] [PubMed]
- Stiber, S.; Hehemann, M.; Carmo, M.; Müller, M.; Ayers, K.E.; Capuano, C.; Danilovic, N.; Morawietz, T.; Biswas, I.; Gazdzicki, P.; et al. Long-Term Operation of Nb-Coated Stainless Steel Bipolar Plates for Proton Exchange Membrane Water Electrolyzers. Adv. Energy Sustain. Res. 2022, 3, 2200024–2200033. [Google Scholar] [CrossRef]
- Rojas, N.; Sánchez-Molina, M.; Sevilla, G.; Amores, E.; Almandoz, E.; Esparza, J.; Cruz Vivas, M.R.; Colominas, C. Coated Stainless Steels Evaluation for Bipolar Plates in PEM Water Electrolysis Conditions. Int. J. Hydrogen Energy 2021, 46, 25929–25943. [Google Scholar] [CrossRef]
- Feng, Q.; Xiao-Zi, Y.; Lui, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Pham, C.V.; Escalera-López, D.; Mayrhofer, K.; Cherevko, S.; Thiele, S. Essentials of High Performance Water Electrolyzers—From Catalyst Layer Materials to Electrode Engineering. Adv. Energy Mater. 2021, 11, 2101998–2102022. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Wang, X.; Fang, B. Conductive Polymer and Nanoparticle-Promoted Polymer Hybrid Coatings for Metallic Bipolar Plates in Proton Membrane Exchange Water Electrolysis. Appl. Sci. 2023, 13, 1244. [Google Scholar] [CrossRef]
- Stiber, S.; Sata, N.; Morawietz, T.; Ansar, S.A.; Jahnke, T.; Lee, J.K.; Bazylak, A.; Fallisch, A.; Gago, A.S.; Friedrich, K.A. A high-performance, durable and low-cost proton exchange membrane electrolyser with stainless steel components. Energy Environ. Sci. 2022, 15, 109–122. [Google Scholar] [CrossRef]
- James, B.D.; Huya-Kouadio, J.; Acevedo, Y.; McNamara, K. Liquid Alkaline Electrolysis Techno-Economic Review, Arlington, VA, USA. Available online: https://www.energy.gov/sites/default/files/2022-02/7-TEA-Liquid%20Alkaline%20Workshop.pdf (accessed on 21 March 2024).
- Gotz, M. STAble and Low Cost Manufactured Bipolar Plates for PEM Fuel Cells. 2015. Available online: www.stampem.eu (accessed on 1 July 2012).
- Wang, H.; Sweikart, M.A.; Turner, J.A. Stainless Steel as Bipolar Plate Material for Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2003, 115, 243–251. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion Exchange Membrane Fuel Cells: Current Status and Remaining Challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Nicotera, I.; Mazzapioda, L.; Aricò, A.S.; Panero, S.; Navarra, M.A. Sulfated Titania as Additive in Nafion Membranes for Water Electrolysis Applications. Int. J. Hydrogen Energy 2017, 42, 27851–27858. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Porembsky, V.I.; Fateev, V.N. Pure Hydrogen Production by PEM Electrolysis for Hydrogen Energy. Int. J. Hydrogen Energy 2006, 31, 171–175. [Google Scholar] [CrossRef]
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.Y. Solid-State Water Electrolysis with an Alkaline Membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, M.F.; Grimaud, A. Water Electrolysers with Closed and Open Electrochemical Systems. Nat. Mater. 2020, 19, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.M.; Chen, C.Y.; Liang, C.H. Comparison of Performance Degradation of High Temperature PEM Fuel Cells with Different Bipolar Plates. Energy 2019, 186, 186–194. [Google Scholar] [CrossRef]
- Dubent, S.; Mazard, A. Characterization and Corrosion Behaviour of Grade 2 Titanium Used in Electrolyzers for Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 15622–15633. [Google Scholar] [CrossRef]



| BPP Materials | Electrolytes | Methods | Surface Modification | ) | ICR (mΩ cm2) | Ref. |
|---|---|---|---|---|---|---|
| Ti | + 5 ppm F− solution at 70 °C with pressured air purging | Pulsed bias arc ion plating | Ti-Ag film | 1 × 10−5 | 2 | [18] |
| Ti | with 2 ppm F−, 70 °C | Pulsed bias arc ion plating | Honeycomb-like nanocomposite Ti-Ag-N films | 1 × 10−5 | 2.3 | [19] |
| Ti | 30 wt% Pt on Vulcan XC-72, 0.5 Pt loading | Electrodeposition | Gold | ― | ― | [20] |
| SS304 Ti | 3 ppm NaF purging with H2, 60 °C | Evaporation processes that use cathodes are known as cathodic | (Ti,Zr) N | 3.10 × 10−7 2.12 × 10−7 | 2.99 2.90 | [26] |
| 446 M ferritic SS | + 2 ppm F− solution, 70 °C | Chemical and thermal treatment | Immersion in the HCl, 50 °C, 5 min | 1.8 × 10−5 | 8 | [27] |
| 446 M ferritic SS | + 2 ppm F− solution at 70 °C | Electrodeposition | Ruthenium oxide | 1 × 10−6 | 2.5 | [28] |
| 446 M ferritic SS | + 2 ppm F− solution at 70 °C | Chemical treatment | Immersion in 5 M NaOH, 1 min | 0.15 × 10−6 | 15.6 | [29] |
| Stainless Steel | Samples Tested under Each SS Category | Corrosion Current Density (Icorr) | Corrosion Potential (Ecorr) | Current Density |
|---|---|---|---|---|
| SS 321 | Bare | 9.13 | 0.06 | 30,117 |
| Ti | 31.6 | 0.02 | 532 | |
| TiN | 0.84 | −0.18 | 412 | |
| Ti/TiN | 2019.00 | −0.22 | 436 | |
| CrN/TiN | 72.92 | −0.01 | 77,985 | |
| TiGr2 | 0.40 | −0.81 | 145 | |
| SS 316L | Bare | 1.47 | −0.39 | 27,423 |
| Ti | 3.79 | −0.04 | 882 | |
| TiN | 145.30 | 0.24 | 549 | |
| Ti/TiN | 139.43 | 0.14 | 564 | |
| CrN/TiN | 15.04 | −0.13 | 69,549 | |
| SS 904L | Bare | 0.78 | −0.16 | 23,298 |
| Ti | 0.02 | −0.11 | 1713 | |
| TiN | 139.70 | −0.26 | 564 | |
| Ti/TiN | 124.81 | −0.21 | 721 | |
| CrN/TiN | 1.24 | −0.35 | 43,844 |
| Manufacturer | Power | Electrolyte | Hydrogen Flow Rate (Nm3·h−1) | Energy Consumption (kWh·Nm−3H2) | Load Range (%) | Series and Operating Pressure |
|---|---|---|---|---|---|---|
| Proton OnSite | No Available | solid polymer electrolyte (SPE) | 0.265–1.05 | 6.7 | 0–100 | S Series 13.8 bar |
| Proton OnSite | No Available | SPE | 2–6 | 6.8–7.3 | 0–100 | H Series 15−30 bar |
| H-TEC Systems | 1–5 kW | SPE | 0.22–1.1 | No available | No available | H-TEC Series-S |
| H-TEC Systems | 225 kW–1 mW | SPE | 13–210 | 4.9 | No available | ME unpressurized 30 bar |
| Areva h2 gen | 80–1600 kVA | SPE | 10–200 | 4.7–5.3 | No available | E series Up to 35 bar |
| Hydrogenics | No Available | SPE | 1–2 | 6.7 | 0–100 | HyLYZER 0–7.9 bar |
| ITM Power | 2 mW | SPE | 0.6–35 | 4.8–5.0 (system) | No available | HPac, HCore, HBox, HFuel 15 bar |
| Siemens | 1.25 mW | SPE | 225 | No available | No available | SILYZER 200 35 bar |
| Green Hydrogen | 4.95 kW | SPE | 1 | No available | 25–100 | P–series/15–50 bar |
| NEL | 0.5–2 mW | SPE | 103–413 | 4.53 | 0–100 | M Series 30 bar |
| Scale | Promising Materials | Limitations and Problems during Operation |
|---|---|---|
| Large-Scale | Titanium, Stainless steel, Graphite | Titanium and stainless steel both have the potential to corrode over time if exposed to an environment that is either extremely acidic or alkaline. The cost of these materials can be quite high when used in large quantities. |
| Industrial | Graphite, Coated titanium, Nickel-based alloys | There is some evidence to suggest that graphite degrades after extended use. Coated titanium can corrode more quickly when exposed to high operating temperatures. |
| Pilot-Scale | Graphite, Carbon composite materials | Restricted Durability: There is a possibility that materials based on graphite will have a restricted ability to withstand long-term use. There is a possibility that the mechanical qualities are insufficient for usage on an industrial scale. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagoraiou, E.; Krishan, S.; Siriwardana, A.; Moschovi, A.M.; Yakoumis, I. Performance of Stainless-Steel Bipolar Plates (SS-BPPs) in Polymer Electrolyte Membrane Water Electrolyser (PEMWE): A Comprehensive Review. Compounds 2024, 4, 252-267. https://doi.org/10.3390/compounds4020013
Zagoraiou E, Krishan S, Siriwardana A, Moschovi AM, Yakoumis I. Performance of Stainless-Steel Bipolar Plates (SS-BPPs) in Polymer Electrolyte Membrane Water Electrolyser (PEMWE): A Comprehensive Review. Compounds. 2024; 4(2):252-267. https://doi.org/10.3390/compounds4020013
Chicago/Turabian StyleZagoraiou, Eirini, Soorya Krishan, Amal Siriwardana, Anastasia Maria Moschovi, and Iakovos Yakoumis. 2024. "Performance of Stainless-Steel Bipolar Plates (SS-BPPs) in Polymer Electrolyte Membrane Water Electrolyser (PEMWE): A Comprehensive Review" Compounds 4, no. 2: 252-267. https://doi.org/10.3390/compounds4020013
APA StyleZagoraiou, E., Krishan, S., Siriwardana, A., Moschovi, A. M., & Yakoumis, I. (2024). Performance of Stainless-Steel Bipolar Plates (SS-BPPs) in Polymer Electrolyte Membrane Water Electrolyser (PEMWE): A Comprehensive Review. Compounds, 4(2), 252-267. https://doi.org/10.3390/compounds4020013