Continuously Interconnected N-Doped Porous Carbon for High-Performance Lithium-Ion Capacitors
Abstract
:1. Introduction
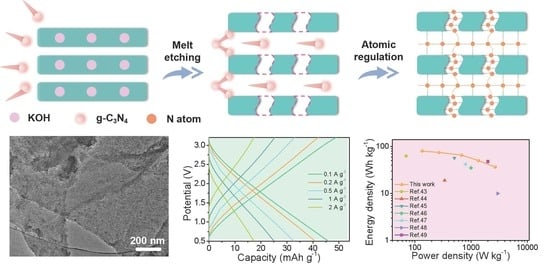
2. Results and Discussion
3. Conclusions
4. Experimental Section
5. Characterization Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Sun, Y.; Tong, Y.; Li, H. FeSb2 Nanoparticles Embedded in 3D Porous Carbon Framework: An Robust Anode Material for Potassium Storage with Long Activation Process. Small 2022, 18, 2201934. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Y.; Zhao, X.; Chen, K.; Bingni, G.; Yang, T.; Zhang, H.; Yang, W.; Chen, J. Tailoring carbon nanomaterials via a molecular scissor. Nano Today 2021, 36, 101033. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, K.; Huang, T.; Shen, G. Recent Advances in Fiber Supercapacitors: Materials, Device Configurations, and Applications. Adv. Mater. 2020, 32, 1901806. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, C.; Zhang, Y.; Lin, L.; He, W.; Xie, Q.; Sa, B.; Wang, L.; Peng, D. A Universal Strategy toward the Precise Regulation of Initial Coulombic Efficiency of Li-Rich Mn-Based Cathode Materials. Adv. Mater. 2021, 33, 2103173. [Google Scholar] [CrossRef]
- Ock, I.W.; Lee, J.; Kang, J.K. Metal-Organic Framework-Derived Anode and Polyaniline Chain Networked Cathode with Mesoporous and Conductive Pathways for High Energy Density, Ultrafast Rechargeable, and Long-Life Hybrid Capacitors. Adv. Energy Mater. 2020, 10, 2001851. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, J.; Tong, Y.; Liu, X.; Sun, Y.; Niu, L.; Li, H. Carbon Hollow Tube-Confined Sb/Sb2S3 Nanorod Fragments as Highly Stable Anodes for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 51066–51077. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R.; et al. Electrode Materials, Electrolytes, and Challenges in Nonaqueous Lithium-Ion Capacitors. Adv. Mater. 2018, 30, 1705670. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Q.; Ding, B.; Wang, J.; Malgras, V.; Jiang, J.; Li, Z.; Chen, S.; Dou, H.; Alshehri, S.M.; et al. Lithium-ion capacitor based on nanoarchitectured polydopamine/graphene composite anode and porous graphene cathode. Carbon 2020, 167, 627–633. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Liu, P.F.; Zhang, H.; He, W.; Xiong, T.F.; Cheng, Y.; Xie, Q.S.; Ma, Y.T.; Zheng, H.F.; Wang, L.S.; Zhu, Z.Z.; et al. Lithium Deficiencies Engineering in Li-Rich Layered Oxide Li1.098Mn0.533Ni0.113Co0.138O2 for High-Stability Cathode. J. Am. Chem. Soc. 2019, 141, 10876–10882. [Google Scholar] [CrossRef]
- Tie, D.; Huang, S.; Wang, J.; Ma, J.; Zhang, J.; Zhao, Y. Hybrid energy storage devices: Advanced electrode materials and matching principles. Energy Storage Mater. 2019, 21, 22–40. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Shellikeri, A.; Wu, Q.; Zheng, J.; Andrei, P.; Zhang, J.-G.; Zheng, J.P. Progress and perspectives on pre-lithiation technologies for lithium ion capacitors. Energy Environ. Sci. 2020, 13, 2341–2362. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Shen, C.; Qin, N.; Zheng, J.; Wu, Q.; Zhang, C.; Zheng, J.P. A universal matching approach for high power-density and high cycling-stability lithium ion capacitor. J. Power Sources 2019, 441, 227211. [Google Scholar] [CrossRef]
- He, W.; Xie, Q.-S.; Lin, J.; Qu, B.-H.; Wang, L.-S.; Peng, D.-L. Mechanisms and applications of layer/spinel phase transition in Li- and Mn-rich cathodes for lithium-ion batteries. Rare Met. 2022, 41, 1456–1476. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.; Jin, Z.; Qiao, X.; Huang, H.; Chu, X.; Xiong, D.; Zhang, H.; Liu, Y.; Yang, W. Hierarchically Divacancy Defect Building Dual-Activated Porous Carbon Fibers for High-Performance Energy-Storage Devices. Adv. Funct. Mater. 2020, 30, 2002580. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Yan, G.; Guo, H.; Li, X.; Wang, Z.; Wang, X.; Yang, Z. Spiral Graphene Coupling Hierarchically Porous Carbon Advances Dual-Carbon Lithium Ion Capacitor. Energy Storage Mater. 2021, 38, 528–534. [Google Scholar] [CrossRef]
- Chen, J.; Yang, B.; Li, H.; Ma, P.; Lang, J.; Yan, X. Candle soot: Onion-like carbon, an advanced anode material for a potassium-ion hybrid capacitor. J. Mater. Chem. A 2019, 7, 9247–9252. [Google Scholar] [CrossRef]
- Li, G.; Yin, Z.; Guo, H.; Wang, Z.; Yan, G.; Yang, Z.; Liu, Y.; Ji, X.; Wang, J. Metalorganic Quantum Dots and Their Graphene-Like Derivative Porous Graphitic Carbon for Advanced Lithium-Ion Hybrid Supercapacitor. Adv. Energy Mater. 2019, 9, 1802878. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Jiang, X.; Qiao, X.; Wang, Y.; Zhao, H.; Pu, B.; Yang, W. Self-assembly defect-regulating superstructured carbon. Energy Storage Mater. 2022, 48, 164–171. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, Y.; Liu, Z.; Yin, Y.; Sun, Y.; Li, H. Fabricating multi-porous carbon anode with remarkable initial coulombic efficiency and enhanced rate capability for sodium-ion batteries. Chin. Chem. Lett. 2023, 34, 107443. [Google Scholar] [CrossRef]
- Zhang, S.S. Dual-Carbon Lithium-Ion Capacitors: Principle, Materials, and Technologies. Batter. Supercaps 2020, 3, 1137–1146. [Google Scholar] [CrossRef]
- Moreno-Fernández, G.; Gómez-Urbano, J.L.; Enterría, M.; Rojo, T.; Carriazo, D. Flat-shaped carbon–graphene microcomposites as electrodes for high energy supercapacitors. J. Mater. Chem. A 2019, 7, 14646–14655. [Google Scholar] [CrossRef] [Green Version]
- Hemmati, S.; Li, G.; Wang, X.; Ding, Y.; Pei, Y.; Yu, A.; Chen, Z. 3D N-doped hybrid architectures assembled from 0D T-Nb2O5 embedded in carbon microtubes toward high-rate Li-ion capacitors. Nano Energy 2019, 56, 118–126. [Google Scholar] [CrossRef]
- Li, J.R.; Zhou, J.; Wang, T.; Chen, X.; Zhang, Y.X.; Wan, Q.; Zhu, J. Covalent sulfur embedding in inherent N,P co-doped biological carbon for ultrastable and high rate lithium-sulfur batteries. Nanoscale 2020, 12, 8991–8996. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Chen, T.; Wang, L.; Ma, L.B.; Hu, Y.; Chen, R.P.; Wang, Y.R.; Wang, C.X.; Yan, W.; Tie, Z.X.; et al. High energy density hybrid lithium-ion capacitor enabled by Co3ZnC@N-doped carbon nanopolyhedra anode and microporous carbon cathode. Energy Storage Mater. 2018, 14, 246–252. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Z.; Han, G.; Zhao, Q.; Lu, G.; Hu, X.; Sun, J.; Wang, R.; Xu, C. Nitrogen-doped activated porous carbon for 4.5 V lithium-ion capacitor with high energy and power density. J. Energy Storage 2022, 47, 103675. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, K.; Chen, M.-S.; Zhang, M.; Shen, Z. One-pot synthesis of N-doped hierarchical porous carbon for high-performance aqueous capacitors in a wide pH range. J. Power Sources 2021, 491, 229587. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, Y.; Tong, Y.; Liu, X.; Sun, Y.; Li, H.; Niu, L. High Capacity and Fast Kinetics of Potassium-Ion Batteries Boosted by Nitrogen-Doped Mesoporous Carbon Spheres. Nano-Micro Lett. 2021, 13, 174. [Google Scholar] [CrossRef]
- Chen, M.; Le, T.; Zhou, Y.; Kang, F.; Yang, Y. Enhanced Electrode Matching Assisted by In Situ Etching and Co-Doping toward High-Rate Dual-Carbon Lithium-Ion Capacitors. ACS Sustain. Chem. Eng. 2021, 9, 10054–10061. [Google Scholar] [CrossRef]
- Liu, X.; Tong, Y.; Wu, Y.; Zheng, J.; Sun, Y.; Niu, L.; Li, H. Synergistically enhanced electrochemical performance using nitrogen, phosphorus and sulfur tri-doped hollow carbon for advanced potassium ion storage device. Chem. Eng. J. 2022, 431, 133986. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Zhang, H.; Jin, L.; Chu, X.; Gu, B.; Huang, H.; Yang, W. Nitrogen, oxygen and sulfur co-doped hierarchical porous carbons toward high-performance supercapacitors by direct pyrolysis of kraft lignin. Carbon 2019, 149, 105–116. [Google Scholar] [CrossRef]
- Xiao, Y.; He, D.; Peng, W.; Chen, S.; Liu, J.; Chen, H.; Xin, S.; Bai, Y. Oxidized-Polydopamine-Coated Graphene Anodes and N,P Codoped Porous Foam Structure Activated Carbon Cathodes for High-Energy-Density Lithium-Ion Capacitors. ACS Appl. Mater. Interfaces 2021, 13, 10336–10348. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yousaf, M.; Wang, Y.; Wang, Z.; Lou, S.; Han, R.P.; Yang, Y.; Cao, A. Nanocable with thick active intermediate layer for stable and high-areal-capacity sodium storage. Nano Energy 2020, 78, 105265. [Google Scholar] [CrossRef]
- Yi, Y.; Zeng, Z.; Lian, X.; Dou, S.; Sun, J. Homologous Nitrogen-Doped Hierarchical Carbon Architectures Enabling Compatible Anode and Cathode for Potassium-Ion Hybrid Capacitors. Small 2022, 18, 2107139. [Google Scholar] [CrossRef]
- Su, F.; Qin, J.; Das, P.; Zhou, F.; Wu, Z.-S. A high-performance rocking-chair lithium-ion battery-supercapacitor hybrid device boosted by doubly matched capacity and kinetics of the faradaic electrodes. Energy Environ. Sci. 2021, 14, 2269–2277. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Sevilla, M. High-surface area carbons from renewable sources with a bimodal micro-mesoporosity for high-performance ionic liquid-based supercapacitors. Carbon 2015, 94, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Gao, Y.; Zhang, C.; Huang, H.; Yan, C.; Chu, X.; Xu, Z.; Wang, Z.; Zhang, H.; Xiao, X.; et al. Highly microporous carbon with nitrogen-doping derived from natural biowaste for high-performance flexible solid-state supercapacitor. J. Colloid Interface Sci. 2019, 548, 322. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Ferrero, G.A.; Sevilla, M. One-pot synthesis of microporous carbons highly enriched in nitrogen and their electrochemical performance. J. Mater. Chem. A 2014, 2, 14439–14448. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Sun, M.; Wang, W.; Chen, C.; Altunkaya, M.; Emwas, A.; Han, Y.; Schwingenschlögl, U.; Alshareef, H.N. Direct Pyrolysis of Supermolecules: An Ultrahigh Edge-Nitrogen Doping Strategy of Carbon Anodes for Potassium-Ion Batteries. Adv. Mater. 2020, 32, 2000732. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Z.; Li, G.R.; Cheng, S.B.; Li, S.; Li, J.D.; Gao, R.; Li, M.; Sy, S.; Deng, Y.P.; et al. Revealing the Rapid Electrocatalytic Behavior of Ultrafine Amorphous Defective Nb2O5-x Nanocluster toward Superior Li-S Performance. ACS Nano 2020, 14, 4849–4860. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, H.; Kim, M.-H.; Mun, Y.; Chun, J.; Ye, Y.; Hwang, J.; Ha, K.-S.; Roh, K.C.; et al. Facile Synthesis of Nb2O5@Carbon Core–Shell Nanocrystals with Controlled Crystalline Structure for High-Power Anodes in Hybrid Supercapacitors. ACS Nano 2015, 9, 7497–7505. [Google Scholar] [CrossRef]
- Arun, N.; Jain, A.; Aravindan, V.; Jayaraman, S.; Ling, W.C.; Srinivasan, M.P.; Madhavi, S. Nanostructured spinel LiNi0.5Mn1.5O4 as new insertion anode for advanced Li-ion capacitors with high power capability. Nano Energy 2015, 12, 69–75. [Google Scholar] [CrossRef]
- Babu, B.; Lashmi, P.; Shaijumon, M. Li-ion capacitor based on activated rice husk derived porous carbon with improved electrochemical performance. Electrochim. Acta 2016, 211, 289–296. [Google Scholar] [CrossRef]
- Chen, Z.; Augustyn, V.; Wen, J.; Zhang, Y.; Shen, M.; Dunn, B.; Lu, Y. High-Performance Supercapacitors Based on Intertwined CNT/V2O5 Nanowire Nanocomposites. Adv. Mater. 2011, 23, 791–795. [Google Scholar] [CrossRef]
- Kim, H.; Cho, M.-Y.; Kim, M.-H.; Park, K.-Y.; Gwon, H.; Lee, Y.; Roh, K.C.; Kang, K. A Novel High-Energy Hybrid Supercapacitor with an Anatase TiO2-Reduced Graphene Oxide Anode and an Activated Carbon Cathode. Adv. Energy Mater. 2013, 3, 1500–1506. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Zhang, W.-B.; Zhao, Y.; Li, K.; Kong, L.-B. Coprecipitation Reaction System Synthesis and Lithium-Ion Capacitor Energy Storage Application of the Porous Structural Bimetallic Sulfide CoMoS4 Nanoparticles. ACS Omega 2018, 3, 8803–8812. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.K.; Lu, C.X.; Zhang, X.; Wan, B.A.; Liu, H.Y.; Xia, M.R.; Gou, H.Y.; Xin, G.Q.; Lian, J.; Zhang, Y.G. Toward ultrafast lithium ion capacitors: A novel atomic layer deposition seeded preparation of Li4Ti5O12/graphene anode. Nano Energy 2017, 36, 46–57. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Jiang, X.; Tong, Q.; Li, H.; Li, J.; Yang, W. Continuously Interconnected N-Doped Porous Carbon for High-Performance Lithium-Ion Capacitors. Nanoenergy Adv. 2022, 2, 303-315. https://doi.org/10.3390/nanoenergyadv2040016
Wang Q, Jiang X, Tong Q, Li H, Li J, Yang W. Continuously Interconnected N-Doped Porous Carbon for High-Performance Lithium-Ion Capacitors. Nanoenergy Advances. 2022; 2(4):303-315. https://doi.org/10.3390/nanoenergyadv2040016
Chicago/Turabian StyleWang, Qing, Xin Jiang, Qijun Tong, Haijian Li, Jie Li, and Weiqing Yang. 2022. "Continuously Interconnected N-Doped Porous Carbon for High-Performance Lithium-Ion Capacitors" Nanoenergy Advances 2, no. 4: 303-315. https://doi.org/10.3390/nanoenergyadv2040016
APA StyleWang, Q., Jiang, X., Tong, Q., Li, H., Li, J., & Yang, W. (2022). Continuously Interconnected N-Doped Porous Carbon for High-Performance Lithium-Ion Capacitors. Nanoenergy Advances, 2(4), 303-315. https://doi.org/10.3390/nanoenergyadv2040016