Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism
Abstract
:1. Introduction
2. Computational Details
3. Results
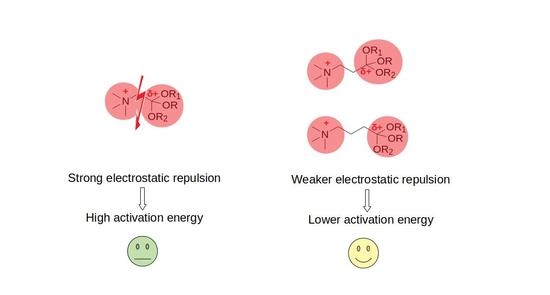
3.1. Formation of Ingold and Watson Protonated Intermediates
3.2. Study of Bachmann-Frapper’s Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X. Valorisation Energétique de la Biomasse Lignocellulosique par Digestion Anaérobie: Prétraitement Fongique Aérobie. Ph.D. Thesis, INSA Lyon, Villeurbanne, France, 18 December 2015. Available online: https://tel.archives-ouvertes.fr/tel-01367705 (accessed on 29 November 2021).
- The Impact of Fossil Fuels on the Environment. Available online: https://fossilfuel.com/the-impact-of-fossil-fuels-on-the-environment/ (accessed on 6 October 2021).
- Pfaltzgraff, L.A.; Clark, J.H. Green Chemistry, Biorefineries and Second-Generation Strategies for Re-Use of Waste: An Overview. In Advances in Biorefineries; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–33. [Google Scholar] [CrossRef]
- Huang, J.; Rozwadowski, K.; Bhinu, V.S.; Schäfer, U.; Hannoufa, A. Manipulation of Sinapine, Choline and Betaine Accumulation in Arabidopsis Seed: Towards Improving the Nutritional Value of the Meal and Enhancing the Seedling Performance under Environmental Stresses in Oilseed Crops. Plant Physiol. Biochem. 2008, 46, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Pérusse, D.; Guégan, J.P.; Rolland, H.; Guilbot, J.; Benvegnu, T. Efficient Solvent-Free Cationization of Alkylpolyglycoside Based Surfactant Compositions Using Natural Glycine Betaine. Green Chem. 2016, 18, 1664–1673. [Google Scholar] [CrossRef]
- Wood, K.V.; Bonham, C.C.; Miles, D.; Rothwell, A.P.; Peel, G.; Wood, B.C.; Rhodes, D. Characterization of Betaines Using Electrospray MS/MS. Phytochemistry 2002, 59, 759–765. [Google Scholar] [CrossRef]
- Qi, L.; Fang, Y.; Wang, Z.; Ma, N.; Jiang, L.; Wang, Y. Synthesis and Physicochemical Investigation of Long Alkylchain Betaine Zwitterionic Surfactant. J. Surfactants Deterg. 2008, 11, 55–59. [Google Scholar] [CrossRef]
- Nsimba, Z.F.; Paquot, M.; Mvumbi, L.G.; Deleu, M. Les dérivés tensioactifs de la glycine bétaïne: Méthodes de synthèse et potentialités d’utilisation. Biotechnol. Agron. Soc. Environ. 2010, 14, 1370–6233. [Google Scholar]
- Sharma, M.; Aguado, R.; Murtinho, D.; Valente, A.J.M.; Ferreira, P.J.T. Novel approach on the synthesis of starch betainate by transesterification. Int. J. Biol. Macromol. 2021, 182, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, Y.; Mohamadou, A.; Boudesocque, S.; Hubert, J.; Plantier-Royon, R.; Dupont, L. Ionic Liquids Derived from Esters of Glycine Betaine: Synthesis and Characterization. J. Mol. Liq. 2015, 207, 60–66. [Google Scholar] [CrossRef]
- Descotes, G. Carbohydrates as Organic Raw Materials II; VCH Publishing: Weinheim, Germany; New York, NY, USA, 1993. [Google Scholar]
- Craig, S.A. Betaine in Human Nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann-Frapper, C.; De Oliveira Vigier, K.; Bachmann, C.; Marinkovic, S.; Estrine, B.; Frapper, G.; Jérôme, F. Elucidation of the Role of Betaine Hydrochloride in Glycerol Esterification: Towards Bio-Based Ionic Building Blocks. Green Chem. 2017, 19, 5647–5652. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Hua, R. Acid-Catalyzed Carboxylic Acid Esterification and Ester Hydrolysis Mechanism: Acylium Ion as a Sharing Active Intermediate via a Spontaneous Trimolecular Reaction Based on Density Functional Theory Calculation and Supported by Electrospray Ionization-Mass Spectrometry. Phys. Chem. Chem. Phys. 2015, 17, 30279–30291. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.B. Organic Chemistry. Modern Theories of Organic Chemistry. D.Sc. Pp. Vii+218. Oxford: Clarendon Press: London: H. Milford, Oxford University Press, 1937. 15s. J. Soc. Chem. Ind. 1938, 57, 418. [Google Scholar] [CrossRef]
- Cukierman, S. Et Tu, Grotthuss! And Other Unfinished Stories. Biochim. Biophys. Acta BBA Bioenerg. 2006, 1757, 876–885. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent Molecular Orbital Methods. XXIII. A Polarization-type Basis Set for Second-row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Schlegel, H.B. Combining Synchronous Transit and Quasi-Newton Methods for Finding Transition States. Isr. J. Chem. 1993, 33, 449–454. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction Path Following in Mass-Weighted Internal Coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, R.J. The Valence-Shell Electron-Pair Repulsion (VSEPR) Theory of Directed Valency. J. Chem. Educ. 1963, 40, 295. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Rablen, P.R. Atomic Charges. J. Org. Chem. 2018, 83, 15463–15469. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Atomic dipole moment corrected hirshfeld population method. J. Theor. Comput. Chem. 2012, 11, 163–183. [Google Scholar] [CrossRef]
- Van Zeist, W.-J.; Bickelhaupt, F. The activation strain model of chemical reactivity. Org. Biomol. Chem. 2010, 8, 3118. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulandou-Koumba, R.D.; Guégan, F.; Ouamba, J.-M.; N’Sikabaka, S.; Frapper, G. Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism. Physchem 2021, 1, 288-296. https://doi.org/10.3390/physchem1030022
Moulandou-Koumba RD, Guégan F, Ouamba J-M, N’Sikabaka S, Frapper G. Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism. Physchem. 2021; 1(3):288-296. https://doi.org/10.3390/physchem1030022
Chicago/Turabian StyleMoulandou-Koumba, Richail Dubien, Frédéric Guégan, Jean-Maurille Ouamba, Samuel N’Sikabaka, and Gilles Frapper. 2021. "Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism" Physchem 1, no. 3: 288-296. https://doi.org/10.3390/physchem1030022
APA StyleMoulandou-Koumba, R. D., Guégan, F., Ouamba, J. -M., N’Sikabaka, S., & Frapper, G. (2021). Acid-Catalyzed Esterification of Betaines: Theoretical Exploration of the Impact of the Carbon Chain Length on the Reaction Mechanism. Physchem, 1(3), 288-296. https://doi.org/10.3390/physchem1030022