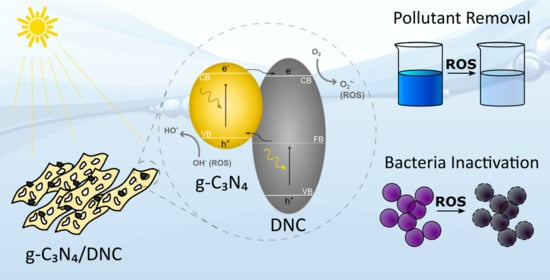
Metal-Free g-C3N4/Nanodiamond Heterostructures for Enhanced Photocatalytic Pollutant Removal and Bacteria Photoinactivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pristine Samples Preparation
2.2. Synthesis of the g-C3N4/DNC Heterojunctions
2.3. Characterization
2.4. Photocatalytic Degradation Study
2.5. Photocatalytic Inactivation of Bacteria
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Liu, Y.; Dong, L.; Shen, C.; Li, F.; Huang, M.; Ma, C.; Yang, B.; An, X.; Sand, W. Recent advances on photocatalytic fuel cell for environmental applications—The marriage of photocatalysis and fuel cells. Sci. Total Environ. 2019, 668, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Du, H.; Liu, Y.-N.; Shen, C.-C.; Xu, A.-W. Nanoheterostructured photocatalysts for improving photocatalytic hydrogen production. Chin. J. Catal. 2017, 38, 1295–1306. [Google Scholar] [CrossRef]
- Stelo, F.; Kublik, N.; Ullah, S.; Wender, H. Recent advances in Bi2MoO6 based Z-scheme heterojunctions for photocatalytic degradation of pollutants. J. Alloys Compd. 2020, 829, 154591. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.-Z.; Yuan, Y.-P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Navarro, R.M.; Alvarez-Galvan, M.C.; de la Mano, J.A.V.; Al-Zahrani, S.; Fierro, J.L.G. A framework for visible-light water splitting. Energy Environ. Sci. 2010, 3, 1865–1882. [Google Scholar] [CrossRef]
- Feil, A.F.; Wender, H.; Gonçalves, R.V. Photovoltaic, Photocatalytic Application, and water Splitting. Nanocatal. Ion. Liq. 2016, 275–294. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Wender, H.; Khan, S.; Melo, M.A. Photocatalytic Water Splitting by Suspended Semiconductor Particles. Nanoenergy 2017, 107–140. [Google Scholar] [CrossRef]
- Nogueira, A.C.; Gomes, L.E.; Ferencz, J.A.P.; Rodrigues, J.E.F.S.; Gonçalves, R.V.; Wender, H. Improved Visible Light Photoactivity of CuBi2O4/CuO Heterojunctions for Photodegradation of Methylene Blue and Metronidazole. J. Phys. Chem. C 2019, 123, 25680–25690. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S.; Hamid, S.B.A. Recent Advances in Heterogeneous Photocatalytic Decolorization of Synthetic Dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light. Appl. Catal. B Environ. 2016, 192, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Peng, T.; Zhang, X.; Li, K.; Ye, L.; Zan, L. Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal. Sci. Technol. 2013, 3, 1253–1260. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y.; Zhao, H.; Wang, H. Fabrication of atomic single layer graphitic-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl. Catal. B Environ. 2014, 152-153, 46–50. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4—Based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.; Guo, X.; Sun, H.; Liu, S.; Tade, M.; Wang, S. Metal-free hybrids of graphitic carbon nitride and nanodiamonds for photoelectrochemical and photocatalytic applications. J. Colloid Interface Sci. 2017, 493, 275–280. [Google Scholar] [CrossRef]
- Silva, G.S.T.; Carvalho, K.T.G.; Lopes, O.; Ribeiro, C. g-C3N4/Nb2O5 heterostructures tailored by sonochemical synthesis: Enhanced photocatalytic performance in oxidation of emerging pollutants driven by visible radiation. Appl. Catal. B Environ. 2017, 216, 70–79. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Facile and green solvothermal synthesis of palladium nanoparticle-nanodiamond-graphene oxide material with improved bifunctional catalytic properties. J. Iran. Chem. Soc. 2017, 14, 2503–2512. [Google Scholar] [CrossRef]
- Tian, Y.; Ge, L.; Wang, K.; Chai, Y. Synthesis of novel MoS2/g-C3N4 heterojunction photocatalysts with enhanced hydrogen evolution activity. Mater. Charact. 2014, 87, 70–73. [Google Scholar] [CrossRef]
- Isberg, J.; Hammersberg, J.; Johansson, E.; Wikström, T.; Twitchen, D.J.; Whitehead, A.J.; Coe, S.E.; Scarsbrook, G.A. High Carrier Mobility in Single-Crystal Plasma-Deposited Diamond. Science 2002, 297, 1670–1672. [Google Scholar] [CrossRef]
- Holt, K.B. Undoped diamond nanoparticles: Origins of surface redox chemistry. Phys. Chem. Chem. Phys. 2010, 12, 2048–2058. [Google Scholar] [CrossRef]
- Hirai, H.; Terauchi, M.; Tanaka, M.; Kondo, K. Estimating band gap of amorphous diamond and nanocrystalline diamond powder by electron energy loss spectroscopy. Diam. Relat. Mater. 1999, 8, 1703–1706. [Google Scholar] [CrossRef]
- Choudhury, S.; Kiendl, B.; Ren, J.; Gao, F.; Knittel, P.; Nebel, C.; Venerosy, A.; Girard, H.; Arnault, J.-C.; Krueger, A.; et al. Combining nanostructuration with boron doping to alter sub band gap acceptor states in diamond materials. J. Mater. Chem. A 2018, 6, 16645–16654. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Xiao, J.; Li, L.; Liu, P.; Wang, C.; Yang, G. Nanodiamond-Embedded p-Type Copper(I) Oxide Nanocrystals for Broad-Spectrum Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2015, 6. [Google Scholar] [CrossRef]
- Su, L.-X.; Huang, Q.-Z.; Lou, Q.; Liu, Z.-Y.; Sun, J.-L.; Zhang, Z.-T.; Qin, S.-R.; Li, X.; Zang, J.-H.; Dong, L.; et al. Effective light scattering and charge separation in nanodiamond@g-C3N4 for enhanced visible-light hydrogen evolution. Carbon 2018, 139, 164–171. [Google Scholar] [CrossRef]
- Yu, S.-J.; Kang, M.-W.; Chang, H.-C.; Chen, K.-M.; Yu, Y.-C. Bright Fluorescent Nanodiamonds: No Photobleaching and Low Cytotoxicity. J. Am. Chem. Soc. 2005, 127, 17604–17605. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Carabineiro, S.A.C.; Buijnsters, J.; Faria, J.; Figueiredo, J.; Silva, A.M.T. Nanodiamond-TiO2Composites for Heterogeneous Photocatalysis. ChemPlusChem 2013, 78, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-D.; Dey, N.K.; Seo, H.O.; Kim, Y.D.; Lim, D.C.; Lee, M. Photocatalytic decomposition of toluene by nanodiamond-supported TiO2 prepared using atomic layer deposition. Appl. Catal. A Gen. 2011, 408, 148–155. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Pastrana-Martínez, L.M.; Silva, A.M.T.; Buijnsters, J.G.; Han, C.; Silva, C.G.; Carabineiro, S.A.C.; Dionysiou, D.D.; Faria, J. Nanodiamond–TiO2 composites for photocatalytic degradation of microcystin-LA in aqueous solutions under simulated solar light. RSC Adv. 2015, 5, 58363–58370. [Google Scholar] [CrossRef]
- Haleem, Y.A.; He, Q.; Liu, D.; Wang, C.; Xu, W.; Gan, W.; Zhou, Y.; Wu, C.; Ding, Y.; Song, L. Facile synthesis of mesoporous detonation nanodiamond-modified layers of graphitic carbon nitride as photocatalysts for the hydrogen evolution reaction. RSC Adv. 2017, 7, 15390–15396. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2018, 214, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, N.; Ong, Y.; Damle, V.G.; Najafi, M.B.H.; Schirhagl, R. Effect of medium and aggregation on antibacterial activity of nanodiamonds. Mater. Sci. Eng. C 2020, 112, 110930. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Makvandi, P.; Zare, E.N.; Tay, F.R.; Niu, L. Advances in Antimicrobial Organic and Inorganic Nanocompounds in Biomedicine. Adv. Ther. 2020, 3. [Google Scholar] [CrossRef]
- Cui, H.; Gu, Z.; Chen, X.; Lin, L.; Wang, Z.; Dai, X.; Yang, Z.; Liu, L.; Zhou, R.; Dong, M. Stimulating antibacterial activities of graphitic carbon nitride nanosheets with plasma treatment. Nanoscale 2019, 11, 18416–18425. [Google Scholar] [CrossRef]
- Wehling, J.; Dringen, R.; Zare, R.N.; Maas, M.; Rezwan, K. Bactericidal Activity of Partially Oxidized Nanodiamonds. ACS Nano 2014, 8, 6475–6483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kalimuthu, S.; Rajasekar, V.; Xu, F.; Yiu, Y.C.; Hui, T.K.C.; Neelakantan, P.; Chu, Z. Biofilm inhibition in oral pathogens by nanodiamonds. Biomater. Sci. 2021, 9, 5127–5135. [Google Scholar] [CrossRef] [PubMed]
- Openda, Y.I.; Ngoy, B.P.; Nyokong, T. Photodynamic Antimicrobial Action of Asymmetrical Porphyrins Functionalized Silver-Detonation Nanodiamonds Nanoplatforms for the Suppression of Staphylococcus aureus Planktonic Cells and Biofilms. Front. Chem. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Openda, Y.I.; Nyokong, T. Detonation nanodiamonds-phthalocyanine photosensitizers with enhanced photophysicochemical properties and effective photoantibacterial activity. Photodiagn. Photodyn. Ther. 2020, 32, 102072. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Mao, Z.; An, N.; Wang, D.; Fahlman, B.D. Ultrathin g-C3N4 nanosheets with an extended visible-light-responsive range for significant enhancement of photocatalysis. RSC Adv. 2017, 7, 2333–2341. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, Z.; Li, C. A comparison of graphitic carbon nitrides synthesized from different precursors through pyrolysis. J. Photochem. Photobiol. A Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Gomes, L.E.; da Silva, M.F.; Gonçalves, R.V.; Machado, G.; Alcantara, G.B.; Caires, A.R.L.; Wender, H. Synthesis and Visible-Light-Driven Photocatalytic Activity of Ta4+ Self-Doped Gray Ta2O5 Nanoparticles. J. Phys. Chem. C 2018, 122, 6014–6025. [Google Scholar] [CrossRef]
- Gomes, L.E.; Nogueira, A.C.; da Silva, M.F.; Plaça, L.F.; Maia, L.J.Q.; Gonçalves, R.V.; Ullah, S.; Khan, S.; Wender, H. Enhanced photocatalytic activity of BiVO4/Pt/PtOx photocatalyst: The role of Pt oxidation state. Appl. Surf. Sci. 2021, 567, 150773. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-Situ-Reduced Synthesis of Ti3+ Self-Doped TiO2/g-C3N4 Heterojunctions with High Photocatalytic Performance under LED Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.; Wu, Y.; Taffa, D.H.; Bottke, P.; Wark, M. Graphitic carbon nitride synthesized by simple pyrolysis: Role of precursor in photocatalytic hydrogen production. New J. Chem. 2019, 43, 6909–6920. [Google Scholar] [CrossRef]
- Piña-Salazar, E.-Z.; Kukobat, R.; Futamura, R.; Hayashi, T.; Toshio, S.; Ōsawa, E.; Kaneko, K. Water-selective adsorption sites on detonation nanodiamonds. Carbon 2018, 139, 853–860. [Google Scholar] [CrossRef]
- Ishida, N.; Kato, K.; Suzuki, N.; Fujimoto, K.; Hagio, T.; Ichino, R.; Kondo, T.; Yuasa, M.; Uetsuka, H.; Fujishima, A.; et al. Preparation of amino group functionalized diamond using photocatalyst and thermal conductivity of diamond/copper composite by electroplating. Diam. Relat. Mater. 2021, 118, 108509. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, Y.; Li, Z.; Yan, S.; Wang, N.; Zou, Z. High-yield synthesis of millimetre-long, semiconducting carbon nitride nanotubes with intense photoluminescence emission and reproducible photoconductivity. Nanoscale 2012, 4, 3687–3692. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2008, 8, 76–80. [Google Scholar] [CrossRef]
- Pina-Salazar, E.-Z.; Urita, K.; Hayashi, T.; Futamura, R.; Vallejos-Burgos, F.; Włoch, J.; Kowalczyk, P.; Wiśniewski, M.; Sakai, T.; Moriguchi, I.; et al. Water Adsorption Property of Hierarchically Nanoporous Detonation Nanodiamonds. Langmuir 2017, 33, 11180–11188. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Nunn, N.; Torelli, M.; McGuire, G.; Shenderova, O. Nanodiamond: A high impact nanomaterial. Curr. Opin. Solid State Mater. Sci. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Aharonovich, I.; Greentree, A.D.; Prawer, S. Diamond photonics. Nat. Photonics 2011, 5, 397–405. [Google Scholar] [CrossRef]
- Hertkorn, J.; Fyta, M. Electronic features of vacancy, nitrogen, and phosphorus defects in nanodiamonds. Electron. Struct. 2019, 1, 025002. [Google Scholar] [CrossRef]
- Li, J.; Yin, Y.; Liu, E.; Ma, Y.; Wan, J.; Fan, J.; Hu, X. In situ growing Bi2MoO6 on g-C3N4 nanosheets with enhanced photocatalytic hydrogen evolution and disinfection of bacteria under visible light irradiation. J. Hazard. Mater. 2017, 321, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Nie, X.; Chen, J.; Jiang, Q.; An, T.; Wong, P.K.; Zhang, H.; Zhao, H.; Yamashita, H. Enhanced visible-light-driven photocatalytic inactivation of Escherichia coli using g-C3N4/TiO2 hybrid photocatalyst synthesized using a hydrothermal-calcination approach. Water Res. 2015, 86, 17–24. [Google Scholar] [CrossRef]
- Tan, H.L.; Wen, X.; Amal, R.; Ng, Y.H. BiVO4 {010} and {110} Relative Exposure Extent: Governing Factor of Surface Charge Population and Photocatalytic Activity. J. Phys. Chem. Lett. 2016, 7, 1400–1405. [Google Scholar] [CrossRef]
- Klimov, V.; Bolívar, P.H.; Kurz, H. Ultrafast carrier dynamics in semiconductor quantum dots. Phys. Rev. B 1996, 53, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hou, J.; Jiang, S.; Lin, Z.; Wang, M.; Che, G. A Z-scheme visible-light-driven Ag/Ag3PO4/Bi2MoO6 photocatalyst: Synthesis and enhanced photocatalytic activity. RSC Adv. 2015, 5, 104815–104821. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Z.; Zheng, G.; Yao, Z.; Mu, J. Superior visible light photocatalytic performance of reticular BiVO4 synthesized via a modified sol–gel method. RSC Adv. 2018, 8, 10654–10664. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Zhang, P.; Li, N.; Sun, Z. Synthesis of Co doped BiVO4 with enhanced visible-light photocatalytic activities. J. Alloys Compd. 2015, 651, 744–748. [Google Scholar] [CrossRef]
- Su, L.-X.; Liu, Z.-Y.; Ye, Y.-L.; Shen, C.-L.; Lou, Q.; Shan, C.-X. Heterostructured boron doped nanodiamonds@g-C3N4 nanocomposites with enhanced photocatalytic capability under visible light irradiation. Int. J. Hydrogen Energy 2019, 44, 19805–19815. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.; Li, Y.; Hu, X.; Wang, S.; Genç, A.; et al. High-yield synthesis and optical properties of g-C3N. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.; Shinde, S.L.; Nanda, K.K. Temperature-Dependent Photoluminescence of g-C3N4: Implication for Temperature Sensing. ACS Appl. Mater. Interfaces 2016, 8, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, R.; Zhao, X.; Chen, Z.; Law, W.K.A.; Zhou, K. A Cobalt-Based Metal–Organic Framework as Cocatalyst on BiVO 4 Photoanode for Enhanced Photoelectrochemical Water Oxidation. ChemSusChem 2018, 11, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, H.; Xu, Y.; Wang, W.; Liang, Q.; Mitsuzaki, N.; Chen, Z. Cobalt–phosphate-modified Mo:BiVO4 mesoporous photoelectrodes for enhanced photoelectrochemical water splitting. J. Mater. Sci. 2019, 54, 10670–10683. [Google Scholar] [CrossRef]
- Ullah, S.; Fayeza; Khan, A.A.; Jan, A.; Aain, S.Q.; Neto, E.P.; Serge-Correales, Y.E.; Parveen, R.; Wender, H.; Rodrigues-Filho, U.P.; et al. Enhanced photoactivity of BiVO4/Ag/Ag2O Z-scheme photocatalyst for efficient environmental remediation under natural sunlight and low-cost LED illumination. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 600, 124946. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kublik, N.; Gomes, L.E.; Plaça, L.F.; Lima, T.H.N.; Abelha, T.F.; Ferencz, J.A.P.; Caires, A.R.L.; Wender, H. Metal-Free g-C3N4/Nanodiamond Heterostructures for Enhanced Photocatalytic Pollutant Removal and Bacteria Photoinactivation. Photochem 2021, 1, 302-318. https://doi.org/10.3390/photochem1020019
Kublik N, Gomes LE, Plaça LF, Lima THN, Abelha TF, Ferencz JAP, Caires ARL, Wender H. Metal-Free g-C3N4/Nanodiamond Heterostructures for Enhanced Photocatalytic Pollutant Removal and Bacteria Photoinactivation. Photochem. 2021; 1(2):302-318. https://doi.org/10.3390/photochem1020019
Chicago/Turabian StyleKublik, Natalya, Luiz E. Gomes, Luiz F. Plaça, Thalita H. N. Lima, Thais F. Abelha, Julio A. P. Ferencz, Anderson R. L. Caires, and Heberton Wender. 2021. "Metal-Free g-C3N4/Nanodiamond Heterostructures for Enhanced Photocatalytic Pollutant Removal and Bacteria Photoinactivation" Photochem 1, no. 2: 302-318. https://doi.org/10.3390/photochem1020019
APA StyleKublik, N., Gomes, L. E., Plaça, L. F., Lima, T. H. N., Abelha, T. F., Ferencz, J. A. P., Caires, A. R. L., & Wender, H. (2021). Metal-Free g-C3N4/Nanodiamond Heterostructures for Enhanced Photocatalytic Pollutant Removal and Bacteria Photoinactivation. Photochem, 1(2), 302-318. https://doi.org/10.3390/photochem1020019