A Personal and Practical Answer from a Clinical Perspective
Abstract
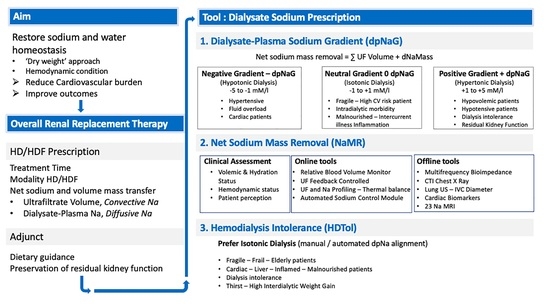
:Dialysate Sodium Prescription from a Personal Clinician Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charra, B.; Calemard, E.; Ruffet, M.; Chazot, C.; Terrat, J.-C.; Vanel, T.; Laurent, G. Survival as an index of adequacy of dialysis. Kidney Int. 1992, 41, 1286–1291. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, R.; Weir, M.R. Dry-weight: A concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Sande, F.M.; van de Wal-Visscher, E.R.; Stuard, S.; Moissl, U.; Kooman, J.P. Using Bioimpedance Spectroscopy to Assess Volume Status in Dialysis Patients. Blood Purif. 2020, 49, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Van Biesen, W.; Williams, J.D.; Covic, A.C.; Fan, S.; Claes, K.; Lichodziejewska-Niemierko, M.; Verger, C.; Steiger, J.; Schoder, V.; Wabel, P.; et al. Fluid status in peritoneal dialysis patients: The European Body Composition Monitoring (EuroBCM) study cohort. PLoS ONE 2011, 6, e17148. [Google Scholar] [CrossRef] [Green Version]
- Canaud, B.; Chazot, C.; Koomans, J.; Collins, A. Fluid and hemodynamic management in hemodialysis patients: Challenges and opportunities. Braz. J. Nephrol. 2019, 41, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Basile, C.; Lomonte, C. A neglected issue in dialysis practice: Haemodialysate. Clin. Kidney J. 2015, 8, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertuglu, L.A.; Demiray, A.; Basile, C.; Afsar, B.; Covic, A.; Kanbay, M. Sodium and ultrafiltration profiling in hemodialysis: A long-forgotten issue revisited. Hemodial. Int. 2021, 25, 433–446. [Google Scholar] [CrossRef]
- Flanigan, M.J. Role of sodium in hemodialysis. Kidney Int. 2000, 58, S72–S78. [Google Scholar] [CrossRef] [Green Version]
- Fujisaki, K.; Joki, N.; Tanaka, S.; Kanda, E.; Hamano, T.; Masakane, I.; Tsuruya, K. Pre-dialysis Hyponatremia and Change in Serum Sodium Concentration During a Dialysis Session Are Significant Predictors of Mortality in Patients Undergoing Hemodialysis. Kidney Int. Rep. 2021, 6, 342–350. [Google Scholar] [CrossRef]
- Lomonte, C.; Basile, C. Do not forget to individualize dialysate sodium prescription. Nephrol. Dial. Transplant. 2011, 26, 1126–1128. [Google Scholar] [CrossRef] [Green Version]
- Basile, C.; Lomonte, C. It is Time to Individualize the Dialysate Sodium Prescription. Semin. Dial. 2015, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Selby, N.M.; Lambie, S.H.; Camici, P.G.; Baker, C.S.; McIntyre, C.W. Occurrence of Regional Left Ventricular Dysfunction in Patients Undergoing Standard and Biofeedback Dialysis. Am. J. Kidney Dis. 2006, 47, 830–841. [Google Scholar] [CrossRef]
- Selby, N.; McIntyre, C.W. A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol. Dial. Transplant. 2006, 21, 1883–1898. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, C.W. Recurrent Circulatory Stress: The Dark Side of Dialysis. Semin. Dial. 2010, 23, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Odudu, A.; McIntyre, C. Volume Is Not the Only Key to Hypertension Control in Dialysis Patients. Nephron 2012, 120, c173–c177. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Stefoni, S.; Petitclerc, T.; Colì, L.; Di Filippo, S.; Andrulli, S.; Fumeron, C.; Frascà, G.M.; Sagripanti, S.; Savoldi, S.; et al. Effect of a plasma sodium biofeedback system applied to HFR on the intradialytic cardiovascular stability. Results from a randomized controlled study. Nephrol. Dial. Transplant. 2012, 27, 3935–3942. [Google Scholar] [CrossRef]
- Robberechts, T.; Allamani, M.; Galloo, X.; Wissing, K.M.; Van Der Niepen, P.; Fongoro, S.; Yattara, H.; Sy, S.; Samaké, M.; Diallo, D.; et al. Individualized Isonatremic and Hyponatremic Dialysate Improves Blood Pressure in Patients with Intradialytic Hypertension: A Prospective Cross-Over Study with 24-h Ambulatory Blood Pressure Monitoring. Open J. Nephrol. 2020, 10, 144–157. [Google Scholar] [CrossRef]
- Raimann, J.G.; Thijssen, S.; Usvyat, L.A.; Levin, N.W.; Kotanko, P. Sodium Alignment in Clinical Practice-Implementation and Implications. Semin. Dial. 2011, 24, 587–592. [Google Scholar] [CrossRef]
- Ságová, M.; Wojke, R.; Maierhofer, A.; Gross, M.; Canaud, B.; Gauly, A. Automated individualization of dialysate sodium concentration reduces intradialytic plasma sodium changes in hemodialysis. Artif. Organs 2019, 43, 1002–1013. [Google Scholar] [CrossRef]
- Ponce, P.; Pinto, B.; Wojke, R.; Maierhofer, A.P.; Gauly, A. Evaluation of intradialytic sodium shifts during sodium controlled hemodialysis. Int. J. Artif. Organs 2020, 43, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Pinter, J.; Chazot, C.; Stuard, S.; Moissl, U.; Canaud, B. Sodium, volume and pressure control in haemodialysis patients for im-proved cardiovascular outcomes. Nephrol Dial. Transpl. 2020, 35 (Suppl. S2), ii23–ii30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canaud, B. A Personal and Practical Answer from a Clinical Perspective. Kidney Dial. 2021, 1, 149-151. https://doi.org/10.3390/kidneydial1020019
Canaud B. A Personal and Practical Answer from a Clinical Perspective. Kidney and Dialysis. 2021; 1(2):149-151. https://doi.org/10.3390/kidneydial1020019
Chicago/Turabian StyleCanaud, Bernard. 2021. "A Personal and Practical Answer from a Clinical Perspective" Kidney and Dialysis 1, no. 2: 149-151. https://doi.org/10.3390/kidneydial1020019
APA StyleCanaud, B. (2021). A Personal and Practical Answer from a Clinical Perspective. Kidney and Dialysis, 1(2), 149-151. https://doi.org/10.3390/kidneydial1020019