Marine Litter Impact on Sandy Beach Fauna: A Review to Obtain an Indication of Where Research Should Contribute More
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
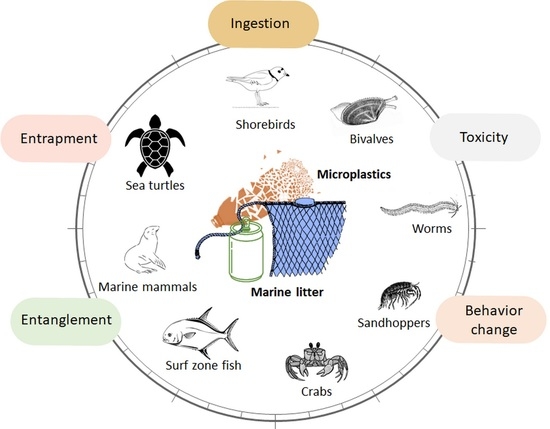
3.1. Overview
3.2. Putative and Diffuse Interactions: A Starting Point
3.3. Ingestion
3.4. Entanglement
3.5. Entrapment
3.6. Individual Trait Changes and Consequences on Populations
3.7. Marine Litter, Beach Fauna, and Active Citizenship Populations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, E.J.; Anderson, S.J.; Harvey, G.R.; Miklas, H.P.; Peck, B.B. Polystyrene Spherules in Coastal Waters. Science 1972, 178, 749–750. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The Global Threat from Plastic Pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.T.; Rangel-Buitrago, N. The Past, Present, and Future of Plastic Pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a Consensus on the Definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Abelouah, M.R.; Ben-Haddad, M.; Rangel-Buitrago, N.; Hajji, S.; El Alem, N.; Ait Alla, A. Microplastics Pollution along the Central Atlantic Coastline of Morocco. Mar. Pollut. Bull. 2022, 174, 113190. [Google Scholar] [CrossRef]
- Gall, S.C.; Thompson, R.C. The Impact of Debris on Marine Life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Kühn, S.; van Franeker, J.A. Quantitative Overview of Marine Debris Ingested by Marine Megafauna. Mar. Pollut. Bull. 2020, 151, 110858. [Google Scholar] [CrossRef]
- McLachlan, A.; Defeo, O. The Ecology of Sandy Shores, 3rd ed.; Elsevier Academic Press: Amsterdan, The Netherlands, 2018. [Google Scholar]
- Fanini, L.; Defeo, O.; Elliott, M. Advances in Sandy Beach Research—Local and Global Perspectives. Estuar. Coast. Shelf Sci. 2020, 234, 106646. [Google Scholar] [CrossRef]
- Morales-Caselles, C.; Viejo, J.; Martí, E.; Fernández, D.G.; Pragnell-Raasch, H.; Gordillo, J.I.G.; Montero, E.; Arroyo, G.M.; Hanke, G.; Salvo, V.S.; et al. An Inshore–Offshore Sorting System Revealed from Global Classification of Ocean Litter. Nat. Sustain. 2021, 4, 484–493. [Google Scholar] [CrossRef]
- Abelouah, M.R.; Ben-Haddad, M.; Alla, A.A.; Rangel-Buitrago, N. Marine Litter in the Central Atlantic Coast of Morocco. Ocean Coast. Manag. 2021, 214, 105940. [Google Scholar] [CrossRef]
- Serra-Gonçalves, C.; Lavers, J.L.; Bond, A.L. Global Review of Beach Debris Monitoring and Future Recommendations. Environ. Sci. Technol. 2019, 53, 12158–12167. [Google Scholar] [CrossRef] [PubMed]
- Krelling, A.P.; Williams, A.T.; Turra, A. Differences in Perception and Reaction of Tourist Groups to Beach Marine Debris That Can Influence a Loss of Tourism Revenue in Coastal Areas. Mar. Policy 2017, 85, 87–99. [Google Scholar] [CrossRef]
- Botero, C.; Anfuso, G.; Williams, A.T.; Zielinski, S.; da Silva, C.P.; Cervantes, O.; Silva, L.; Cabrera, J.A. Reasons for Beach Choice: European and Caribbean Perspectives. J. Coast. Res. 2013, 65, 880–885. [Google Scholar] [CrossRef]
- Harris, L.; Campbell, E.E.; Nel, R.; Schoeman, D. Rich Diversity, Strong Endemism, but Poor Protection: Addressing the Neglect of Sandy Beach Ecosystems in Coastal Conservation Planning. Divers. Distrib. 2014, 20, 1120–1135. [Google Scholar] [CrossRef]
- Costa, L.L.; Zalmon, I.R. Macroinvertebrates as Umbrella Species on Sandy Beaches. Biol. Conserv. 2021, 253, 108922. [Google Scholar] [CrossRef]
- Wenneker, B.; Oosterbaan, L. Guideline for Monitoring Marine Litter on the Beaches in the OSPAR Maritime Area; OSPAR Commission: London, UK, 2010. [Google Scholar]
- Ribbink, T.; Baleta, T.; Martin, S.; Mbongwa, N.; Bray, D. Guideline to Marine Litter Monitoring; Western Indian Ocean Marine Science Association: Zanzibar, Tanzania, 2019; 80p. [Google Scholar]
- Jepsen, E.M.; de Bruyn, P.J.N. Pinniped Entanglement in Oceanic Plastic Pollution: A Global Review. Mar. Pollut. Bull. 2019, 145, 295–305. [Google Scholar] [CrossRef]
- Fanini, L.; Plaiti, W.; Papageorgiou, N. Environmental Education: Constraints and Potential as Seen by Sandy Beach Researchers. Estuar. Coast. Shelf Sci. 2019, 218, 173–178. [Google Scholar] [CrossRef]
- Ivar do Sul, J.A.; Costa, M.F. Marine Debris Review for Latin America and the Wider Caribbean Region: From the 1970s until Now, and Where Do We Go from Here? Mar. Pollut. Bull. 2007, 54, 1087–1104. [Google Scholar] [CrossRef]
- Ben-Haddad, M.; De-la-Torre, G.E.; Abelouah, M.R.; Hajji, S.; Alla, A.A. Personal Protective Equipment (PPE) Pollution Associated with the COVID-19 Pandemic along the Coastline of Agadir, Morocco. Sci. Total Environ. 2021, 798, 149282. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.L.; da Costa, M.F.; da Zalmon, I.R. Macroinvertebrates as Biomonitors of Pollutants on Natural Sandy Beaches: Overview and Meta-Analysis. Environ. Pollut. 2021, 275, 116629. [Google Scholar] [CrossRef]
- Costa, L.L.; Fanini, L.; Zalmon, I.R.; Defeo, O. Macroinvertebrates as Indicators of Human Disturbances: A Global Review. Ecol. Indic. 2020, 118, 106764. [Google Scholar] [CrossRef]
- Bessa, F.; Ratcliffe, N.; Otero, V.; Sobral, P.; Marques, J.C.; Waluda, C.M.; Trathan, P.N.; Xavier, J.C. Microplastics in Gentoo Penguins from the Antarctic Region. Sci. Rep. 2019, 9, 14191. [Google Scholar] [CrossRef] [PubMed]
- Torres-Zevallos, U.; Ayala, F.; Guzman, R.; García, M.; Iannacone, J. Ingesta de Desecho Antrópico En Otaria Flavescens (Carnivora: Otariidae) En Playa “San Pedro”, Lurín, Perú. Biotempo 2020, 17, 163–171. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Oakleaf, J.R.; Theobald, D.M.; Baruch-Mordo, S.; Kiesecker, J. Managing the Middle: A Shift in Conservation Priorities Based on the Global Human Modification Gradient. Glob. Change Biol. 2019, 25, 811–826. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling 2020, R package version 3.4-5.
- Bivand, R.; Keitt, T.; Rowlingson, B. Rgdal: Bindings for the “Geospatial” Data Abstraction Library 2021.
- Lourenço, P.M.; Serra-Gonçalves, C.; Ferreira, J.L.; Catry, T.; Granadeiro, J.P. Plastic and Other Microfibers in Sediments, Macroinvertebrates and Shorebirds from Three Intertidal Wetlands of Southern Europe and West Africa. Environ. Pollut. 2017, 231, 123–133. [Google Scholar] [CrossRef]
- Battisti, C.; Kroha, S.; Kozhuharova, E.; De Michelis, S.; Fanelli, G.; Poeta, G.; Pietrelli, L.; Cerfolli, F. Fishing Lines and Fish Hooks as Neglected Marine Litter: First Data on Chemical Composition, Densities, and Biological Entrapment from a Mediterranean Beach. Environ. Sci. Pollut. Res. 2019, 26, 1000–1007. [Google Scholar] [CrossRef]
- Fanini, L.; Defeo, O.; Elliott, M.; Paragkamian, S.; Pinna, M.; Salvo, V.S. Coupling Beach Ecology and Macroplastics Litter Studies: Current Trends and the Way Ahead. Mar. Pollut. Bull. 2021, 173, 112951. [Google Scholar] [CrossRef]
- Fowler, C.W. Marine Debris and Northern Fur Seals: A Case Study. Mar. Pollut. Bull. 1987, 18, 326–335. [Google Scholar] [CrossRef]
- Stewart, B.S.; Yochem, P.K. Entanglement of Pinnipeds in Synthetic Debris and Fishing Net and Line Fragments at San Nicolas and San Miguel Islands, California, 1978–1986. Mar. Pollut. Bull. 1987, 18, 336–339. [Google Scholar] [CrossRef]
- Shaughnessy, P.D. Entanglement of Cape Fur Seals with Man-Made Objects. Mar. Pollut. Bull. 1980, 11, 332–336. [Google Scholar] [CrossRef]
- Feldkamp, S.D.; Costa, D.P.; Dekrey, G.K. Energetic and Behavioral Effects of Net Entanglement on Juvenile Northern Fur Seals, Callorhinus ursinus. Fish. Bull. 1989, 87, 85–94. [Google Scholar]
- Fowler, C.W.; Merrick, R.; Baker, J.D. Studies of the Population Level Effects of Entanglement on Northern Fur Seals. (Callorhinus ursinus). In U.S. Department of Commerce, Noaa Technical Memorandum NMFS, NOAA-TM-NMFS-SWFC-154, Proceedings of the Second International Conference on Marine Debris, 2–7 April 1989, Honolulu, HI, USA; pp. 453–474; Shomura, R.S., Godfrey, M.L., Eds.; U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service: Washington, DC, USA, 1990; p. 22. [Google Scholar]
- Raum-suryan, K.L. Entanglement of Steller Sea Lions in Marine Debris and Fishing Gear on the Central Oregon Coast from 2005–2009. Oceans 2022, 3, 319–330. [Google Scholar] [CrossRef]
- Widmer, W.M.; Hennemann, M.C. Marine Debris in the Island of Santa Catarina, South Brazil: Spatial Patterns, Composition, and Biological Aspects. J. Coast. Res. 2010, 26, 993–1000. [Google Scholar] [CrossRef]
- Foster-Smith, J.; Birchenough, A.; Evans, S.; Prince, J. Human Impacts on Cable Beach, Broome (Western Australia). Coast. Manag. 2007, 35, 181–194. [Google Scholar] [CrossRef]
- Suciu, M.C.; Tavares, D.C.; Costa, L.L.; Silva, M.C.L.; Zalmon, I.R. Evaluation of Environmental Quality of Sandy Beaches in Southeastern Brazil. Mar. Pollut. Bull. 2017, 119, 133–142. [Google Scholar] [CrossRef]
- Tavares, D.C.; Moura, J.F.; Ceesay, A.; Merico, A. Density and Composition of Surface and Buried Plastic Debris in Beaches of Senegal. Sci. Total Environ. 2020, 737, 139633. [Google Scholar] [CrossRef]
- Suciu, M.C.; Tavares, D.C.; Zalmon, I.R. Comparative Evaluation of Crustaceans as Bioindicators of Human Impact on Brazilian Sandy Beaches. J. Crustac. Biol. 2018, 38, 420–428. [Google Scholar] [CrossRef]
- Costa, L.L.; Zalmon, I.R. Multiple Metrics of the Ghost Crab Ocypode quadrata (Fabricius, 1787) for Impact Assessments on Sandy Beaches. Estuar. Coast. Shelf Sci. 2019, 218, 237–245. [Google Scholar] [CrossRef]
- Arueira, V.F.; Zalmon, I.R.; Costa, L.L. Is the ghost crab’s feeding behavior a good early indicator of human pressure in sandy beaches? Reg. Stud. Mar. Sci. 2022, 53, 102381. [Google Scholar] [CrossRef]
- Barboza, C.A.; Mattos, G.; Soares-Gomes, A.; Zalmon, I.R.; Costa, L.L. Low Densities of the Ghost Crab Ocypode quadrata Related to Large Scale Human Modification of Sandy Shores. Front. Mar. Sci. 2021, 8, 589542. [Google Scholar] [CrossRef]
- Costa, L.L.; Rangel, D.F.; Zalmon, I.R. Evidence of Marine Debris Usage by the Ghost Crab Ocypode quadrata (Fabricius, 1787). Mar. Pollut. Bull. 2018, 128, 438–445. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Lucrezi, S.; Connolly, R.M.; Peterson, C.H.; Gilby, B.L.; Maslo, B.; Olds, A.D.; Walker, S.J.; Leon, J.X.; Huijbers, C.M.; et al. Human Threats to Sandy Beaches: A Meta-Analysis of Ghost Crabs Illustrates Global Anthropogenic Impacts. Estuar. Coast. Shelf Sci. 2016, 169, 56–73. [Google Scholar] [CrossRef]
- Neves, F.M.; Benvenutti, C.E. The Ghost Crab Ocypode Quadrata (Fabricius, 1787) as a Potential Indicator of Anthropic Impact along the Rio Grande Do Sul Coast, Brazil. Biol. Conserv. 2006, 33, 431–435. [Google Scholar] [CrossRef]
- Ugolini, A.; Ungherese, G.; Ciofini, M.; Lapucci, A.; Camaiti, M. Microplastic Debris in Sandhoppers. Estuar. Coast. Shelf Sci. 2013, 129, 19–22. [Google Scholar] [CrossRef]
- Costa, L.L.; Madureira, J.F.; Di Beneditto, A.P.M.; Zalmon, I.R. Interaction of the Atlantic Ghost Crab with Marine Debris: Evidence from an in Situ Experimental Approach. Mar. Pollut. Bull. 2019, 140, 603–609. [Google Scholar] [CrossRef]
- Iannilli, V.; Di Gennaro, A.; Lecce, F.; Sighicelli, M.; Falconieri, M.; Pietrelli, L.; Poeta, G.; Battisti, C. Microplastics in Talitrus saltator (Crustacea, Amphipoda): New Evidence of Ingestion from Natural Contexts. Environ. Sci. Pollut. Res. 2018, 25, 28725–28729. [Google Scholar] [CrossRef]
- Hodgson, D.J.; Bréchon, A.L.; Thompson, R.C. Ingestion and Fragmentation of Plastic Carrier Bags by the Amphipod Orchestia gammarellus: Effects of Plastic Type and Fouling Load. Mar. Pollut. Bull. 2018, 127, 154–159. [Google Scholar] [CrossRef]
- Gomes, T.T.; Gheler-Costa, C.; Rinaldi, C.A.; Santana, W. Natural Diet of Ocypode quadrata (Fabricius, 1787) (Crustacea, Decapoda, Brachyura) from the Northern Coast of São Paulo, Brazil. Papéis Avulsos Zool. 2019, 59, e20195957. [Google Scholar] [CrossRef]
- Costa, L.L.; Arueira, V.F.; da Costa, M.F.; Di Beneditto, A.P.M.; Zalmon, I.R. Can the Atlantic Ghost Crab Be a Potential Biomonitor of Microplastic Pollution of Sandy Beaches Sediment? Mar. Pollut. Bull. 2019, 145, 5–13. [Google Scholar] [CrossRef] [PubMed]
- do Vale, J.G.; Costa Barrilli, G.H.; Chahad-Ehlers, S.; Branco, J.O. Factors Influencing the Feeding Habits of the Ghost Crab Ocypode quadrata (Fabricius, 1787) on Subtropical Sandy Beaches. Estuar. Coast. Shelf Sci. 2022, 269, 107817. [Google Scholar] [CrossRef]
- Gusmão, F.; Domenico, M.D.; Amaral, A.C.Z.; Martínez, A.; Gonzalez, B.C.; Worsaae, K.; Ivar do Sul, J.A.; Cunha Lana, P. da In Situ Ingestion of Microfibres by Meiofauna from Sandy Beaches. Environ. Pollut. 2016, 216, 584–590. [Google Scholar] [CrossRef]
- Kang, T.; Kim, D.; Oh, J.H. Ingestion of Microplastics by Free-Living Marine Nematodes, Especially Enoplolaimus Spp., in Mallipo Beach, South Korea. Plankton Benthos Res. 2021, 16, 109–117. [Google Scholar] [CrossRef]
- Horn, D.; Miller, M.; Anderson, S.; Steele, C. Microplastics Are Ubiquitous on California Beaches and Enter the Coastal Food Web through Consumption by Pacific Mole Crabs. Mar. Pollut. Bull. 2019, 139, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haddad, M.; Rida, M.; Hajji, S.; De-la-torre, G.E.; Abou, H.; Rangel-buitrago, N.; Ait, A. The Wedge Clam Donax trunculus L., 1758 as a Bioindicator of Microplastic Pollution. Mar. Pollut. Bull. 2022, 178, 113607. [Google Scholar] [CrossRef]
- Narmatha Sathish, M.; Immaculate Jeyasanta, K.; Patterson, J. Monitoring of Microplastics in the Clam Donax cuneatus and Its Habitat in Tuticorin Coast of Gulf of Mannar (GoM), India. Environ. Pollut. 2020, 266, 115219. [Google Scholar] [CrossRef]
- Xu, X.; Wong, C.Y.; Tam, N.F.Y.; Lo, H.S.; Cheung, S.G. Microplastics in Invertebrates on Soft Shores in Hong Kong: Influence of Habitat, Taxa and Feeding Mode. Sci. Total Environ. 2020, 715, 136999. [Google Scholar] [CrossRef]
- Baechler, B.R.; Granek, E.F.; Hunter, M.V.; Conn, K.E. Microplastic Concentrations in Two Oregon Bivalve Species: Spatial, Temporal, and Species Variability. Limnol. Oceanogr. Lett. 2020, 5, 54–65. [Google Scholar] [CrossRef]
- Truchet, D.M.; López, A.D.F.; Ardusso, M.G.; Rimondino, G.N.; Buzzi, N.S.; Malanca, F.E.; Spetter, C.V.; Severini, M.D.F. Microplastics in Bivalves, Water and Sediments from a Touristic Sandy Beach of Argentina. Mar. Pollut. Bull. 2021, 173. [Google Scholar] [CrossRef]
- Suebpala, W.; Yeemin, T.; Sutthacheep, M.; Sangiamdee, D.; Phaoduang, S.; Sangsawang, L.; Rangseethampanya, P.; Wongnutpranont, A. Comparing the Abundance of Microplastics in the Wedge Shell, Donax Semigranosus from Ta Kuan and Leam Mea Phim Beaches, Rayong Province. Ramkhamhaeng Int. J. Sci. Technol. 2018, 1, 25–32. [Google Scholar]
- Mayoma, B.S.; Sørensen, C.; Shashoua, Y.; Khan, F.R. Microplastics in Beach Sediments and Cockles (Anadara antiquata) along the Tanzanian Coastline. Bull. Environ. Contam. Toxicol. 2020, 105, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Bendell, L.I.; LeCadre, E.; Zhou, W. Use of Sediment Dwelling Bivalves to Biomonitor Plastic Particle Pollution in Intertidal Regions; A Review and Study. PLoS ONE 2020, 15, 1–21. [Google Scholar] [CrossRef]
- Tosetto, L.; Brown, C.; Williamson, J.E. Microplastics on Beaches: Ingestion and Behavioural Consequences for Beachhoppers. Mar. Biol. 2016, 163, 4–13. [Google Scholar] [CrossRef]
- Horn, D.A.; Granek, E.F.; Steele, C.L. Effects of Environmentally Relevant Concentrations of Microplastic Fibers on Pacific Mole Crab (Emerita analoga) Mortality and Reproduction. Limnol. Oceanogr. Lett. 2020, 5, 74–83. [Google Scholar] [CrossRef]
- Tlili, S.; Jemai, D.; Brinis, S.; Regaya, I. Microplastics Mixture Exposure at Environmentally Relevant Conditions Induce Oxidative Stress and Neurotoxicity in the Wedge Clam Donax trunculus. Chemosphere 2020, 258, 127344. [Google Scholar] [CrossRef]
- Xu, X.Y.; Lee, W.T.; Chan, A.K.Y.; Lo, H.S.; Shin, P.K.S.; Cheung, S.G. Microplastic Ingestion Reduces Energy Intake in the Clam Atactodea striata. Mar. Pollut. Bull. 2017, 124, 798–802. [Google Scholar] [CrossRef]
- Lavers, J.L.; Bond, A.L. Exceptional and Rapid Accumulation of Anthropogenic Debris on One of the World’s Most Remote and Pristine Islands. Proc. Natl. Acad. Sci. USA 2017, 114, 6052–6055. [Google Scholar] [CrossRef]
- Edo, C.; Tamayo-Belda, M.; Martínez-Campos, S.; Martín-Betancor, K.; González-Pleiter, M.; Pulido-Reyes, G.; García-Ruiz, C.; Zapata, F.; Leganés, F.; Fernández-Piñas, F.; et al. Occurrence and Identification of Microplastics along a Beach in the Biosphere Reserve of Lanzarote. Mar. Pollut. Bull. 2019, 143, 220–227. [Google Scholar] [CrossRef]
- Caro, T. Conservation by Proxy: Indicator, Umbrella, Keystone, Flagship, and Other Surrogates, 2nd ed.; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Nationwide Monitoring of Microplastics in Bivalves from the Coastal Environment of Korea. Environ. Pollut. 2021, 270, 116175. [Google Scholar] [CrossRef]
- Tlili, S.; Mouneyrac, C. The Wedge Clam Donax trunculus as Sentinel Organism for Mediterranean Coastal Monitoring in a Global Change Context. Reg. Environ. Change 2019, 19, 995–1007. [Google Scholar] [CrossRef]
- Dantas, N.C.F.M.; Duarte, O.S.; Ferreira, W.C.; Ayala, A.P.; Rezende, C.F.; Feitosa, C.V. Plastic Intake Does Not Depend on Fish Eating Habits: Identification of Microplastics in the Stomach Contents of Fish on an Urban Beach in Brazil. Mar. Pollut. Bull. 2020, 153, 110959. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, P.M. Comparative Feeding Ecology of Shorebirds Wintering at Banc d’ Arguin, Mauritania. Estuaries Coasts 2015, 39, 855–865. [Google Scholar] [CrossRef]
- Ranatunga, R.R.M.K.P.; Wijetunge, D.S.; Karunarathna, K.P.R. Microplastics in Beach Sand and Potential Contamination of Planktivorous Fish Sardinella Gibbosa Inhabiting in Coastal Waters of Negombo, Sri Lanka. Sri Lanka J. Aquat. Sci. 2021, 26, 37–54. [Google Scholar] [CrossRef]
- McGregor, S.; Strydom, N.A. Feeding Ecology and Microplastic Ingestion in Chelon richardsonii (Mugilidae) Associated with Surf Diatom Anaulus australis Accumulations in a Warm Temperate South African Surf Zone. Mar. Pollut. Bull. 2020, 158, 111430. [Google Scholar] [CrossRef] [PubMed]
- de Amorim, A.L.A.; Ramos, J.A.A.; Júnior, M.N. Ingestion of Microplastic by Ontogenetic Phases of Stellifer brasiliensis (Perciformes, Sciaenidae) from the Surf Zone of Tropical Beaches. Mar. Pollut. Bull. 2020, 158, 111214. [Google Scholar] [CrossRef] [PubMed]
- Fanini, L.; Piscart, C.; Pranzini, E.; Kerbiriou, C.; Le Viol, I.; Pétillon, J. The Extended Concept of Littoral Active Zone Considering Soft Sediment Shores as Social-Ecological Systems, and an Application to Brittany (North-Western France). Estuar. Coast. Shelf Sci. 2021, 250, 107148. [Google Scholar] [CrossRef]
- Chua, E.M.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Clarke, B.O. Assimilation of Polybrominated Diphenyl Ethers from Microplastics by the Marine Amphipod, Allorchestes Compressa. Environ. Sci. Technol. 2014, 48, 8127–8134. [Google Scholar] [CrossRef]
- Scopetani, C.; Cincinelli, A.; Martellini, T.; Lombardini, E.; Ciofini, A.; Fortunati, A.; Pasquali, V.; Ciattini, S.; Ugolini, A. Ingested Microplastic as a Two-Way Transporter for PBDEs in Talitrus Saltator. Environ. Res. 2018, 167, 411–417. [Google Scholar] [CrossRef]
- Galgani, L.; Beiras, R.; Galgani, F.; Panti, C.; Borja, A. Editorial: “Impacts of Marine Litter”. Front. Mar. Sci. 2019, 6, 208. [Google Scholar] [CrossRef]
- Derraik, J.G.B. The Pollution of the Marine Environment by Plastic Debris: A Review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Nelms, S.E.; Duncan, E.M.; Broderick, A.C.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Lindeque, P.K.; Godley, B.J. Plastic and Marine Turtles: A Review and Call for Research. ICES J. Mar. Sci. 2016, 73, 165–181. [Google Scholar] [CrossRef] [Green Version]
- Gündoğdu, S.; Yeşilyurt, İ.N.; Erbaş, C. Potential Interaction between Plastic Litter and Green Turtle Chelonia mydas during Nesting in an Extremely Polluted Beach. Mar. Pollut. Bull. 2019, 140, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Triessnig, P.; Roetzer, A.; Stachowitsch, M. Beach Condition and Marine Debris: New Hurdles for Sea Turtle Hatchling Survival. Chelonian Conserv. Biol. 2012, 11, 68–77. [Google Scholar] [CrossRef]
- Lavers, J.L.; Sharp, P.B.; Stuckenbrock, S.; Bond, A.L. Entrapment in Plastic Debris Endangers Hermit Crabs. J. Hazard. Mater. 2020, 387, 121703. [Google Scholar] [CrossRef]
- Romiti, F.; Pietrangeli, E.; Battisti, C.; Carpaneto, G.M. Quantifying the Entrapment Effect of Anthropogenic Beach Litter on Sand-dwelling Beetles According to the EU Marine Strategy Framework Directive. J. Insect Conserv. 2021, 25, 441–452. [Google Scholar] [CrossRef]
- Aloy, A.B.; Vallejo, B.M.; Juinio-Meñez, M.A. Increased Plastic Litter Cover Affects the Foraging Activity of the Sandy Intertidal Gastropod Nassarius pullus. Mar. Pollut. Bull. 2011, 62, 1772–1779. [Google Scholar] [CrossRef]
- Costa, L.L.; Zalmon, I.R. Surf Zone Fish Diet as an Indicator of Environmental and Anthropogenic Influences. J. Sea Res. 2017, 128, 61–75. [Google Scholar] [CrossRef]
- Fujisaki, I.; Lamont, M.M. The Effects of Large Beach Debris on Nesting Sea Turtles. J. Exp. Mar. Bio. Ecol. 2016, 482, 33–37. [Google Scholar] [CrossRef]
- Özdilek, H.G.; Yalçin-Özdilek, Ş.; Ozaner, F.S.; Sönmez, B. Impact of Accumulated Beach Litter on Chelonia Mydas L. 1758 (Green Turtle) Hatchlings of the Samandaǧ Coast, Hatay, Turkey. Fresenius Environ. Bull. 2006, 15, 95–103. [Google Scholar]
- Zielinski, S.; Botero, C.M.; Yanes, A. To Clean or Not to Clean? A Critical Review of Beach Cleaning Methods And. Mar. Pollut. Bull. 2019, 139, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Fanini, L.; Cantarino, C.M.; Scapini, F. Relationships between the Dynamics of Two Talitrus saltator Populations and the Impacts of Activities Linked to Tourism. Oceanologia 2005, 47, 93–112. [Google Scholar]
- Dugan, J.E.; Hubbard, D.M.; Mccrary, M.; Hubbard, D.M.; Mccrary, M.D.; Pierson, M.O. The Response of Macrofauna Communities and Shorebirds to Macrophyte Wrack Subsidies on Exposed Sandy Beaches of Southern California. Estuar. Coast. Shelf Sci. 2003, 58, 25–40. [Google Scholar] [CrossRef]
- Fanini, L.; Lowry, J.K. Comparing Methods Used in Estimating Biodiversity on Sandy Beaches: Pitfall vs. Quadrat Sampling. Ecol. Indic. 2016, 60, 358–366. [Google Scholar] [CrossRef]
- Williams, A.T.; Rangel-Buitrago, N.G.; Anfuso, G.; Cervantes, O.; Botero, C.M. Litter Impacts on Scenery and Tourism on the Colombian North Caribbean Coast. Tour. Manag. 2016, 55, 209–224. [Google Scholar] [CrossRef]
- Riechers, M.; Brunner, B.P.; Dajka, J.C.; Dușe, I.A.; Lübker, H.M.; Manlosa, A.O.; Sala, J.E.; Schaal, T.; Weidlich, S. Leverage Points for Addressing Marine and Coastal Pollution: A Review. Mar. Pollut. Bull. 2021, 167, 112263. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Carracher, L.K.; Porch, N.; Connolly, R.M.; Olds, D.; Gilby, B.L.; Ekanayake, K.B.; Maslo, B.; Weston, M.A. The Early Shorebird Will Catch Fewer Invertebrates on Trampled Sandy Beaches. PLoS ONE 2016, 11, e0161905. [Google Scholar] [CrossRef]
- Battisti, C.; Poeta, G.; Pietrelli, L.; Acosta, A.T.R. An Unexpected Consequence of Plastic Litter Clean-up on Beaches: Too Much Sand Might Be Removed. Environ. Pract. 2016, 18, 242–246. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Thiel, M. The Contribution of Citizen Scientists to the Monitoring of Marine Litter. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Eds.; Springer: Berlin, Germany, 2015; pp. 433–451. [Google Scholar]
- Camins, E.; de Haan, W.P.; Salvo, V.; Canals, M.; Raffard, A.; Sanchez-vidal, A. Paddle Surfing for Science on Microplastic Pollution. Sci. Total Environ. 2020, 709, 1–10. [Google Scholar] [CrossRef]
- de Haan, W.P.; Uviedo, O.; Ballesteros, M.; Canales, Í.; Curto, X.; Guart, M.; Higueras, S.; Molina, A.; Sanchez-Vidal, A. Floating Microplastic Loads in the Nearshore Revealed through Citizen Science. Environ. Res. Letters. 2022, 17, 045018. [Google Scholar] [CrossRef]
- Hong, S.; Lee, J.; Jang, Y.C.; Kim, Y.J.; Kim, H.J.; Han, D.; Hong, S.H.; Kang, D.; Shim, W.J. Impacts of Marine Debris on Wild Animals in the Coastal Area of Korea. Mar. Pollut. Bull. 2013, 66, 117–124. [Google Scholar] [CrossRef] [PubMed]
- European Commission Guidance on Monitoring Marine Litter in European Seas 2013, 128.
- Fanini, L.; Costa, L.L.; Zalmon, I.R.; Riechers, M. Social and Ecological Elements for a Perspective Approach to Citizen Science on the Beach. Front. Ecol. Evol. 2021, 9, 694487. [Google Scholar] [CrossRef]
- Maya-Jariego, I.; Martínez-Alba, I.; Alieva, D. “Plenty of Black Money”: Netnography of Illegal Recreational Underwater Fishing in Southern Spain. Mar. Policy 2021, 126, 104411. [Google Scholar] [CrossRef]
- Reed, C. Dawn of the Plasticene Age. New Sci. 2015, 225, 28–32. [Google Scholar] [CrossRef]
- Jacobson, S.K.; Morales, N.A.; Chen, B.; Soodeen, R.; Moulton, M.P.; Jain, E. Love or Loss: Effective Message Framing to Promote Environmental Conservation. Appl. Environ. Educ. Commun. 2019, 18, 252–265. [Google Scholar] [CrossRef]
- Carrasco, A.; Pulgar, J.; Quintanilla-Ahumada, D.; Perez-Venegas, D.; Quijón, P.A.; Duarte, C. The Influence of Microplastics Pollution on the Feeding Behavior of a Prominent Sandy Beach Amphipod, Orchestoidea tuberculata (Nicolet, 1849). Mar. Pollut. Bull. 2019, 145, 23–27. [Google Scholar] [CrossRef]
- Majer, A.P.; Vedolin, M.C.; Turra, A. Plastic Pellets as Oviposition Site and Means of Dispersal for the Ocean-Skater Insect Halobates. Mar. Pollut. Bull. 2012, 64, 1143–1147. [Google Scholar] [CrossRef]
- Henderson, J.R. A pre-and post-MARPOL Annex V summary of Hawaiian monk seal entanglements and marine debris accumulation in the Northwestern Hawaiian Islands, 1982–1998. Mar. Pollut. Bull. 2001, 42, 584–589. [Google Scholar] [CrossRef]
- Page, B.; McKenzie, J.; McIntosh, R.; Baylis, A.; Morrissey, A.; Calvert, N.; Haase, M.B.; Berris, M.; Dowie, D.; Shaughnessy, P.D.; et al. Entanglement of Australian Sea Lions and New Zealand Fur Seals in Lost Fishing Gear and Other Marine Debris Before and After Government and Industry Attempts to Reduce the Problem. Mar. Pollut. Bull. 2004, 49, 33–42. [Google Scholar] [CrossRef]
- Pemberton, D.; Brothers, N.P.; Kirkwood, R. Entanglement of Australian Fur Seals in Man-made Debris in Tasmanian Waters. Wildl. Res. 1992, 19, 151–159. [Google Scholar] [CrossRef]
- Harcourt, R.; Aurioles, D.; Sanchez, J. Entanglement of California Sea Lions at Los Islotes, Baja California Sur, México. Mar. Mammal Sci. 1994, 10, 122–125. [Google Scholar] [CrossRef]
- Hanni, K.D.; Pyle, P. Entanglement of Pinnipeds in Synthetic Materials at South-east Farallon Island, California, 1976–1998. Mar. Pollut. Bull. 2000, 40, 1076–1081. [Google Scholar] [CrossRef]
- Hofmeyr, G.; De Maine, M.; Beste, M.; Kirkman, S.; Pistorius, P.; Makhado, A. Entanglement of Pinnipeds at Marion Island, Southern Ocean: 1991–2001. Aust. Mammal. 2002, 24, 141–146. [Google Scholar] [CrossRef]
- Hofmeyr, G.; Bester, M.N. Entanglement of Pinnipeds at Marion Island. Afr. J. Mar. Sci. 2002, 24, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Arnould, J.P.; Croxall, J.P. Trends in entanglement of Antarctic Fur Seals (Arctocephalus gazella) in Man-Made Debris at South Georgia. Mar. Pollut. Bull. 1995, 30, 707–712. [Google Scholar] [CrossRef]
- Croxall, J.P.; Rodwell, S.; Boyd, I.L. Entanglement in Man-Made Debris of Antarctic Fur Seals at Bird Island, South Georgia. Mar. Mammal Sci. 1990, 6, 221–233. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]





| Taxonomic Group | Species Name | Marine Litter Type | Impact Type | Reference |
|---|---|---|---|---|
| Bivalves | Anadara antiquata | Microplastics | Ingestion | [68] |
| Donax trunculus | [62] | |||
| Donax cuneatus | [63] | |||
| Donax semigranosus | [67] | |||
| Donax spp. | [64] | |||
| Amarilladesma mactroides | [66] | |||
| Siliqua patula | [65] | |||
| Ruditapes philippinarum | [77] | |||
| Nutallia obscurata | [69] | |||
| Venerupis phillippinarum | ||||
| Neopycnodonte cochlear | Fishing lines and hooks | Entrapment | [33] | |
| (Other species) | Microplastics | |||
| Atactoidea striata | Metabolic effects | [73] | ||
| Gastropods | Nassarius pullus | Macroplastics | Behavioral traits | [94] |
| Crustaceans | Talitrus saltator (Sandhoppers) | Microplastics | Ingestion | [52,54] |
| Ocypode quadrata (Ghost crabs) | Beach debris | Homing | [49] | |
| Microplastics and macroplastics | Ingestion | [56,57,58] | ||
| Macroplastics | Behavior traits | [49,53] | ||
| Ocypode quadrata | Marine litter | Reduction in abundance | [41,45] | |
| Atlantorchestoidea brasiliensis | Marine litter | [45] | ||
| Excirolana braziliensis | ||||
| Emerita brasiliensis | ||||
| Platorchestia smithi | Microplastics | Behavior traits/shredding | [55,70,115] | |
| Orchestoidea tuberculata | ||||
| Orchestia gammarellus | ||||
| Allorchestes compressa | Microplastics | Vehiculation of POPs | [85,86] | |
| Emerita analoga (Mole Crabs) | Microplastics | Ingestion | [61,71] | |
| Coenobita perlatus (Strawberry Hermit Crab) | Macroplastics | Entrapment | [92] | |
| Polychaetes | Saccocirrus papillocercus | Microfibers | Ingestion | [59] |
| Saccocirrus pussicus | ||||
| Claudrilus ovarium | ||||
| Meiodrilus gracilis | ||||
| (other species) | ||||
| Nematodes | Enoplolaimus spp. | Microplastics | Ingestion | [60] |
| Insects | Isomira sp. | Beach litter | Entrapment | [93] |
| Mogulones aubei | ||||
| Ammobius rufus | ||||
| Opatrum obesum | ||||
| (Other species) | ||||
| Halobates micans | Plastic pellets | Oviposition | [116] | |
| Fish | Chelon rischardsonii | Microplastics | Ingestion | [79,81,82,83] |
| Opisthonema oglinum | ||||
| Bagre marinus Cathorops spixii Sciades herzbergii | ||||
| Chloroscombrus chrysurus | ||||
| Conodon nobilis | ||||
| Haemulopsis corvinaeformis | ||||
| Stellifer brasiliensis | ||||
| Genidens genidens | ||||
| Arius grandicassis | ||||
| Menticirrhus americanus | ||||
| Polydactylus virginicus | ||||
| Sardinella gibbosa | ||||
| (Other species) | ||||
| Trachinotus spp. | Marine litter | Behavior traits | [95] | |
| Reptiles | Sea turtles | Beach litter | Behavior traits | [96] |
| Chelonia mydas (Sea turtles) | Macroplastics | Entanglement | [90] | |
| Beach litter | Behavior traits | [97] | ||
| Sea turtles | Marine litter | Entanglement | [91] | |
| Entrapment | ||||
| Birds | Arenaria interpres | Microplastics | Ingestion | [32] |
| Calidris alba | ||||
| Calidris alpina | ||||
| Calidris canutus | ||||
| Calidris ferruginea | ||||
| (Other species) | ||||
| Mammals | Arctocephalus ssp (fur seal) | Marine litter (mainly fishing gear) | Entanglement | [35,36,37,38,117,118,119,120,121,122,123,124,125] |
| Arctocephalus forsteri | ||||
| Neophorca cinerea | ||||
| Zalophus californianus | ||||
| Monachus schauinslandi | ||||
| Arctocephalus ssp. | ||||
| Mirounga leonine | ||||
| Callorhinus ursinus | ||||
| Arctocephalus pusillus | ||||
| Arctocephalus gazella | ||||
| Arctocephalus ssp | ||||
| Callorhinus ursinus | Fishing gear | Behavioral, energetic and population changes | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, L.L.; Fanini, L.; Ben-Haddad, M.; Pinna, M.; Zalmon, I.R. Marine Litter Impact on Sandy Beach Fauna: A Review to Obtain an Indication of Where Research Should Contribute More. Microplastics 2022, 1, 554-571. https://doi.org/10.3390/microplastics1030039
Costa LL, Fanini L, Ben-Haddad M, Pinna M, Zalmon IR. Marine Litter Impact on Sandy Beach Fauna: A Review to Obtain an Indication of Where Research Should Contribute More. Microplastics. 2022; 1(3):554-571. https://doi.org/10.3390/microplastics1030039
Chicago/Turabian StyleCosta, Leonardo Lopes, Lucia Fanini, Mohamed Ben-Haddad, Maurizio Pinna, and Ilana Rosental Zalmon. 2022. "Marine Litter Impact on Sandy Beach Fauna: A Review to Obtain an Indication of Where Research Should Contribute More" Microplastics 1, no. 3: 554-571. https://doi.org/10.3390/microplastics1030039
APA StyleCosta, L. L., Fanini, L., Ben-Haddad, M., Pinna, M., & Zalmon, I. R. (2022). Marine Litter Impact on Sandy Beach Fauna: A Review to Obtain an Indication of Where Research Should Contribute More. Microplastics, 1(3), 554-571. https://doi.org/10.3390/microplastics1030039