Green Extraction of Oleoresin from Pink Pepper Fruits: Effect of Experimental Conditions and Characterization
Abstract
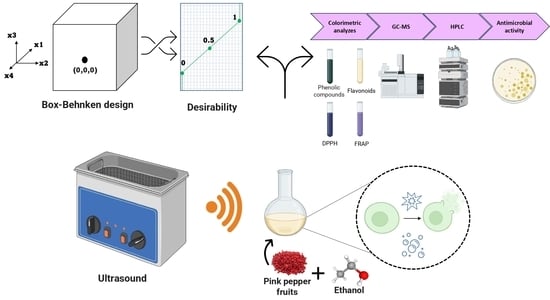
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Extractions Assays
2.4. Oleoresin Characterization
2.5. Antimicrobial Activity
2.6. Data Analysis
3. Results and Discussion
3.1. Ultrasound Assisted Extraction
3.1.1. Effect of Temperature
3.1.2. Effect of Ultrasound Power Intensity
3.1.3. Effect of Sample to Solvent Ratio
3.1.4. Effect of Time
3.1.5. Maximization
3.2. UAE and Soxhlet Extraction Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fagundes, M.B.; Ballus, C.A.; Perceval Soares, V.; de Freitas Ferreira, D.; Sena Vaz Leães, Y.; Sasso Robalo, S.; Guidetti Vendruscolo, R.; Bastianello Campagnol, P.C.; Smanioto Barin, J.; Cichoski, A.J.; et al. Characterization of olive oil flavored with Brazilian pink pepper (Schinus terebinthifolius Raddi) in different maceration processes. Food Res. Int. 2020, 137, 109593. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Oliveira, V.S.; Augusta, I.M.; da Braz, M.V.C.; Riger, C.J.; Prudêncio, E.R.; Sawaya, A.C.H.F.; Sampaio, G.R.; da Silva Torres, E.A.F.; Saldanha, T. Aroeira fruit (Schinus terebinthifolius Raddi) as a natural antioxidant: Chemical constituents, bioactive compounds and in vitro and in vivo antioxidant capacity. Food Chem. 2020, 315, 126274. [Google Scholar] [CrossRef]
- Oliveira, M.B.S.; Valentim, I.B.; Rocha, T.S.; Santos, J.C.; Pires, K.S.N.; Tanabe, E.L.L.; Borbely, K.S.C.; Borbely, A.U.; Goulart, M.O.F. Schinus terebenthifolius Raddi extracts: From sunscreen activity toward protection of the placenta to Zika virus infection, new uses for a well-known medicinal plant. Ind. Crops Prod. 2020, 152, 112503. [Google Scholar] [CrossRef]
- Dannenberg, G.S.; Funck, G.D.; da Silva, W.P.; Fiorentini, Â.M. Essential oil from pink pepper (Schinus terebinthifolius Raddi): Chemical composition, antibacterial activity and mechanism of action. Food Control 2019, 95, 115–120. [Google Scholar] [CrossRef]
- Silva, M.M.C.; Bezerra de Araújo Neto, J.; Lucas dos Santos, A.T.; Morais Oliveira-Tintino, C.D.; Araújo, A.C.J.; Freitas, P.R.; Silva, L.E.; Amaral, W.; Deschamps, C.; Azevedo, F.R.; et al. Antibiotic-potentiating activity of the Schinus terebinthifolius Raddi essential oil against MDR bacterial strains. Plants 2023, 12, 1587. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Oliveira, H.; Fernandes, I.; Simal-Gandara, J.; Perez-Gregorio, R. Recent advances in extracting phenolic compounds from food and their use in disease prevention and as cosmetics. Crit. Rev. Food Sci. Nutr. 2020, 61, 1130–1151. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, W.; Zhao, S.; Yang, X.; Xu, W.; Guo, M.; Xu, E.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted extraction of lipids as food components: Mechanism, solvent, feedstock, quality evaluation and coupled technologies—A review. Trends Food Sci. Technol. 2022, 122, 83–96. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of ultrasound-assisted extraction (UAE) process for the recovery of bioactive compounds from bitter gourd using response surface methodology (RSM). Food Bioprod. Process 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Md Norodin, N.S.; Sarkawi, N.S.; Hamzah, M.H.S.; Mohd Nasir, H.; Abang Zaidel, D.N.; Che Yunus, M.A.; Md Salleh, L. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Pagano, I.; del Pilar Sánchez-Camargo, A.; Mendiola, J.A.; Campone, L.; Cifuentes, A.; Rastrelli, L.; Ibañez, E. Selective extraction of high-value phenolic compounds from distillation wastewater of basil (Ocimum basilicum L.) by pressurized liquid extraction. Electrophoresis 2018, 39, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef] [PubMed]
- Rebelatto, E.A.; Rodrigues, L.G.G.; Rudke, A.R.; Andrade, K.S.; Ferreira, S.R.S. Sequential green-based extraction processes applied to recover antioxidant extracts from pink pepper fruits. J. Supercrit. Fluids 2020, 166, 105034. [Google Scholar] [CrossRef]
- Uquiche, E.; Del Valle, J.M.; Ortiz, J. Supercritical carbon dioxide extraction of red pepper (Capsicum annuum L.) oleoresin. J. Food Eng. 2004, 65, 55–66. [Google Scholar] [CrossRef]
- Andrade, K.S.; Poncelet, D.; Ferreira, S.R.S. Sustainable extraction and encapsulation of pink pepper oil. J. Food Eng. 2017, 204, 38–45. [Google Scholar] [CrossRef]
- Raspe, D.T.; Ciotta, S.R.; Zorzenon, M.R.T.; Dacome, A.S.; da Silva, C.; Milani, P.G.; da Costa, S.C. Ultrasound-assisted extraction of compounds from Stevia leaf pretreated with ethanol. Ind. Crops Prod. 2021, 172, 114035. [Google Scholar] [CrossRef]
- Haiyan, Z.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem. 2007, 100, 1544–1551. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Santos, K.A.; Klein, E.J.; Silva, C.; Silva, E.A.; Cardozo-Filho, L. Extraction of vetiver (Chrysopogon zizanioides) root oil by supercritical CO2, pressurized-liquid, and ultrasound-assisted methods and modeling of supercritical extraction kinetics. J. Supercrit. Fluids 2019, 150, 30–39. [Google Scholar] [CrossRef]
- Stevanato, N.; Silva, C. Radish seed oil: Ultrasound-assisted extraction using ethanol as solvent and assessment of its potential for ester production. Ind. Crops Prod. 2019, 132, 283–291. [Google Scholar] [CrossRef]
- Yin, G.; Dang, Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Zaidan, U.H.; Zen, N.I.M.; Amran, N.A.; Shamsi, S.; Gani, S.S.A. Biochemical evaluation of phenolic compounds and steviol glycoside from Stevia rebaudiana extracts associated with in vitro antidiabetic potential. Biocatal. Agric. Biotechnol. 2019, 18, 101049. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Mello, B.T.F.; Stevanato, N.; Cardozo Filho, L.; Silva, C. Pressurized liquid extraction of radish seed oil using ethanol as solvent: Effect of pretreatment on seeds and process variables. J. Supercrit. Fluids 2021, 176, 105307. [Google Scholar] [CrossRef]
- Santos Júnior, O.O.; Montanher, P.F.; Bonafé, E.G.; do Prado, I.N.; Maruyama, S.A.; Matsushita, M.; Visentainer, J.V. A Simple, Fast and Efficient Method for Transesterification of Fatty Acids in Foods Assisted by Ultrasound Energy. J. Braz. Chem. Soc. 2014, 25, 1712–1719. [Google Scholar] [CrossRef]
- Costa, A.J.N.; Stevanato, N.; Raspe, D.T.; Cardozo-Filho, L.; Silva, C. Valorization of coffee bean husk via pressurized liquid extraction for production of physicochemical extract. J. Chem. Technol. Biotechnol. 2023, 1–9. [Google Scholar] [CrossRef]
- CLSI M07-A8; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 8th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009.
- Pinc, M.M.; Dalmagro, M.; Cruz, E.A.P.; Donadel, G.; Thomaz, R.T.; da Silva, C.; Macruz, P.D.; Jacomassi, E.; Gasparotto Junior, A.; Hoscheid, J.; et al. Extraction Methods, Chemical Characterization, and In Vitro Biological Activities of Plinia cauliflora (Mart.) Kausel Peels. Pharmaceuticals 2023, 16, 1173. [Google Scholar] [CrossRef] [PubMed]
- Reche, C.; Rosselló, C.; Dalmau, E.; Eim, V.; Simal, S. Quantification of microstructural changes in artichoke by-products by image analysis after high-power ultrasound-assisted extraction of bioactive compounds. LWT 2022, 171, 114127. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Manikandan, S.; Vigna Nivetha, C.; Dinesh, R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2017, 10, S1145–S1157. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K. Microwave and ultrasound assisted extraction of phytocompounds from black jamun pulp: Kinetic and thermodynamics characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102913. [Google Scholar] [CrossRef]
- Raj, G.V.S.B.; Dash, K.K. Ultrasound-assisted extraction of phytocompounds from dragon fruit peel: Optimization, kinetics and thermodynamic studies. Ultrason. Sonochem. 2020, 68, 105180. [Google Scholar] [CrossRef]
- Rohilla, S.; Chutia, H.; Marboh, V.; Mahanta, C.L. Ultrasound and supercritical fluid extraction of phytochemicals from purple tamarillo: Optimization, comparison, kinetics, and thermodynamics studies. Appl. Food Res. 2022, 2, 100210. [Google Scholar] [CrossRef]
- Belhachat, D.; Mekimene, L.; Belhachat, M.; Ferradji, A.; Aid, F. Application of response surface methodology to optimize the extraction of essential oil from ripe berries of Pistacia lentiscus using ultrasonic pretreatment. J. Appl. Res. Med. Aromat. Plants 2018, 9, 132–140. [Google Scholar] [CrossRef]
- Imtiaz, F.; Ahmed, D.; Abdullah, R.H.; Ihsan, S. Green extraction of bioactive compounds from Thuja orientalis leaves using microwave- and ultrasound-assisted extraction and optimization by response surface methodology. Sustain. Chem. Pharm. 2023, 35, 101212. [Google Scholar] [CrossRef]
- Rouhani, M. Modeling and optimization of ultrasound-assisted green extraction and rapid HPTLC analysis of stevioside from Stevia rebaudiana. Ind. Crops Prod. 2019, 132, 226–235. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
- Boudries, H.; Nabet, N.; Chougui, N.; Souagui, S.; Loupassaki, S.; Madani, K.; Dimitrov, K. Optimization of ultrasound-assisted extraction of antioxidant phenolics from Capparis spinosa flower buds and LC–MS analysis. J. Food Meas. Charact. 2019, 13, 2241–2252. [Google Scholar] [CrossRef]
- Santos, K.A.; Silva, E.A.; Silva, C. Ultrasound-assisted extraction of favela (Cnidoscolus quercifolius) seed oil using ethanol as a solvent. J. Food Process Preserv. 2021, 45, e15497. [Google Scholar] [CrossRef]
- Liao, J.; Guo, Z.; Yu, G. Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason. Sonochem. 2021, 76, 105661. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.P.; Bolanho, B.C.; Stevanato, N.; Massa, T.B.; Silva, C. Ultrasound-assisted extraction of red beet pigments (Beta vulgaris L.): Influence of operational parameters and kinetic modeling. J. Food Process Preserv. 2020, 44, e14762. [Google Scholar] [CrossRef]
- Shewale, S.P.; Kapadia, M.; Rathod, V.K. Intensification of total phenolic compounds extraction from Azadirachta indica (Neem) leaves by ultrasound. Chem. Eng. Process 2022, 181, 109099. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Dabie, K.; Abassah-Oppong, S.; Peprah-Yamoah, E. Optimization of ultrasound-assisted extraction of phenolic antioxidants from turkey berry (Solanum torvum Sw) fruits using response surface methodology. J. Appl. Res. Med. Aromat. Plants 2022, 30, 100387. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef]
- More, P.R.; Arya, S.S. Intensification of bio-actives extraction from pomegranate peel using pulsed ultrasound: Effect of factors, correlation, optimization and antioxidant bioactivities. Ultrason. Sonochem. 2021, 72, 105423. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Petitcolas, E.; Maache-Rezzoug, Z.; Chemat, F.; Rezzoug, S.A. Impact of ultrasound on solid-liquid extraction of phenolic compounds from maritime pine sawdust waste. Kinetics, optimization and large scale experiments. Ultrason. Sonochem. 2016, 28, 230–239. [Google Scholar] [CrossRef]
- López-Bascón-Bascon, M.A.; Luque de Castro, M.D. Soxhlet Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef]
- Stevanato, N.; Mello, B.T.F.; Saldaña, M.D.A.; Cardozo-Filho, L.; Silva, C. Production of ethyl esters from forage radish seed: An integrated sequential route using pressurized ethanol and ethyl acetate. Fuel 2023, 332, 126075. [Google Scholar] [CrossRef]
- de Araujo Gomes, R.B.; de Souza, E.S.; Gerhardt Barraqui, N.S.; Tosta, C.L.; Nunes, A.P.F.; Schuenck, R.P.; Ruas, F.G.; Ventura, J.A.; Filgueiras, P.R.; Kuster, R.M. Residues from the Brazilian pepper tree (Schinus terebinthifolia Raddi) processing industry: Chemical profile and antimicrobial activity of extracts against hospital bacteria. Ind. Crops Prod. 2020, 143, 111430. [Google Scholar] [CrossRef]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Social Security. Report on Health and Social Subjects: Diet and Cardiovascular Disease; Committee on Medical Aspects of Food Policy. Report of the Panel on Diet in Relation to Cardiovascular Disease; Her Majesty’s Stationery Office: London, UK, 1984. [Google Scholar]
- Vieira, J.S.; Oliveira, V.S.; Carneiro, M.J.; Silva, T.L.; Augusta, I.M.; Carvalho, M.G.; Sawaya, A.C.H.F.; Saldanha, T. Phenolic composition and insights into the use of pink pepper (Schinus terebentifolius Raddi) fruit against lipid oxidation in food systems. Food Biosci. 2023, 53, 102556. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Rahaman, M.M.; Islam, T.; Bappi, M.H.; Sikder, M.I.; Hossain, K.N.; Akter, F.; Prottay, A.A.S.; Rokonuzzman, M.; Gürer, E.S.; et al. Neurobiological effects of gallic acid: Current perspectives. Chin. Med. 2023, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Qu, T.-B.; Zhai, K.; Ding, J.; Hai, Y.; Zhou, J.-L. Gallic acid can play a chondroprotective role against AGE-induced osteoarthritis progression. J. Orthop. Sci. 2015, 20, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Kataoka, T.; Hayashi, T.; Hasegawa, M.; Ishi, Y.; Hibasami, H. Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO 205 cell lines. Oncol. Rep. 2000, 7, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Cheemanapalli, S.; Mopuri, R.; Golla, R.; Anuradha, C.M.; Chitta, S.K. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, X.; Wu, W.; Qin, R. Effect of Syringic acid on antioxidant biomarkers and associated inflammatory markers in mice model of asthma. Drug Dev. Res. 2018, 80, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, Y.; Araki, Y.; Konoike, T. Stereoselective Total Synthesis of (-)-α-Eudesmol, a P/Q-Type Calcium Channel Blocker. Org. Lett. 2001, 2001, 1452–1454. [Google Scholar] [CrossRef]
- Acharya, B.; Chaijaroenkul, W.; Na-Bangchang, K. Therapeutic potential and pharmacological activities of β-eudesmol. Chem. Biol. Drug Des. 2021, 97, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Mevy, J.P.; Bessiere, J.M.; Dherbomez, M.; Millogo, J.; Viano, J. Chemical composition and some biological activities of the volatile oils of a chemotype of Lippia chevalieri Moldenke. Food Chem. 2007, 101, 682–685. [Google Scholar] [CrossRef]
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative Study of Subcritical Water and Microwave-Assisted Extraction Techniques Impact on the Phenolic Compounds and 5-Hydroxymethylfurfural Content in Pomegranate Peel. Plant Foods Hum. Nutr. 2020, 75, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, Y.; Ping, X.; Liu, J.; Wang, X.; Qin, N. Chemical composition and antibacterial activity of ethyl acetate extract of Astragalus membranaceus aerial parts. J. Food Saf. 2021, 42, e12947. [Google Scholar] [CrossRef]
- Lafraxo, S.; Zouirech, O.; El Barnossi, A.; Chelouati, T.; Chebaibi, M.; Chebbac, K.; Nafidi, H.-A.; Salamatullah, A.M.; Bourhia, M.; Aboul-Soud, M.A.M.; et al. Promising antioxidant and antimicrobial effects of essential oils extracted from fruits of Juniperus thurifera: In vitro and in silico investigations. Open Chem. 2023, 21, 20220332. [Google Scholar] [CrossRef]
- Kart, N.N.B.; Günal, B.; Mutlu, D.; Doğan, N.M.; Arslan, Ş.; Semiz, G. Evaluating Antibiofilm, Cytotoxic and Apoptotic Activities of Scutellaria brevibracteata subsp. brevibracteata Essential Oil. Chem. Biodivers. 2023, 20, e202300878. [Google Scholar] [CrossRef]
- Benkhaira, N.; Zouine, N.; Fadil, M.; Koraichi, S.I.; El Hachlafi, N.; Jeddi, M.; Lachkar, M.; Fikri-Benbrahim, K. Application of mixture design for the optimum antibacterial action of chemically-analyzed essential oils and investigation of the antiadhesion ability of their optimal mixtures on 3D printing material. Bioprinting 2023, 34, e00299. [Google Scholar] [CrossRef]
- Prakash, V.; Kaur, H.; Bala, R. Chemical Constituents, Antimicrobial and Anti-Inflammatory Activities of Anaphalis Triplinervis Essential Oil. Nat. Vol. Essent. Oil 2023, 10, 72–83. [Google Scholar] [CrossRef]

| Independent Variable | Coded Variable | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | X1 | 30 | 45 | 60 |
| Ultrasonic power intensity (%) | X2 | 0 | 50 | 100 |
| Sample to solvent ratio (g mL−1) | X3 | 1:10 | 1:15 | 1:20 |
| Time (min) | X4 | 15 | 30 | 45 |
| Run | Temperature (°C) | Ultrasonic Power Intensity (%) | Sample to Solvent Ratio (g mL−1) | Time (min) | YGE (wt%) | YPC (mg GAE * per g Fruit) |
|---|---|---|---|---|---|---|
| 1 | 30 (−1) | 0 (−1) | 1:15 (0) | 30 (0) | 11.47 | 1.59 ± 0.03 |
| 2 | 60 (1) | 0 (−1) | 1:15 (0) | 30 (0) | 21.73 | 3.85 ± <0.01 |
| 3 | 30 (−1) | 100 (1) | 1:15 (0) | 30 (0) | 17.94 | 2.64 ± 0.02 |
| 4 | 60 (1) | 100 (1) | 1:15 (0) | 30 (0) | 23.44 | 5.07 ± <0.01 |
| 5 | 45 (0) | 50 (0) | 1:10 (−1) | 15 (−1) | 13.98 | 2.06 ± 0.02 |
| 6 | 45 (0) | 50 (0) | 1:20 (1) | 15 (−1) | 16.02 | 2.38 ± <0.01 |
| 7 | 45 (0) | 50 (0) | 1:10 (−1) | 45 (1) | 21.32 | 3.84 ± <0.01 |
| 8 | 45 (0) | 50 (0) | 1:20 (1) | 45 (1) | 23.34 | 4.15 ± 0.01 |
| 9 | 45 (0) | 50 (0) | 1:15 (0) | 30 (0) | 17.64 | 2.58 ± 0.01 |
| 10 | 30 (−1) | 50 (0) | 1:15 (0) | 15 (−1) | 14.03 | 1.71 ± <0.01 |
| 11 | 60 (1) | 50 (0) | 1:15 (0) | 15 (−1) | 17.07 | 3.34 ± <0.01 |
| 12 | 30 (−1) | 50 (0) | 1:15 (0) | 45 (1) | 14.65 | 2.17 ± 0.01 |
| 13 | 60 (1) | 50 (0) | 1:15 (0) | 45 (1) | 26.50 | 4.84 ± 0.01 |
| 14 | 45 (0) | 0 (−1) | 1:10 (−1) | 30 (0) | 15.84 | 3.13 ± 0.01 |
| 15 | 45 (0) | 100 (1) | 1:10 (−1) | 30 (0) | 18.28 | 3.75 ± <0.01 |
| 16 | 45 (0) | 0 (−1) | 1:20 (1) | 30 (0) | 16.43 | 2.57 ± <0.01 |
| 17 | 45 (0) | 100 (1) | 1:20 (1) | 30 (0) | 19.54 | 3.91 ± 0.03 |
| 18 | 45 (0) | 50 (0) | 1:15 (0) | 30 (0) | 17.40 | 2.51 ± 0.01 |
| 19 | 30 (−1) | 50 (0) | 1:10 (−1) | 30 (0) | 14.02 | 1.95 ± 0.02 |
| 20 | 60 (1) | 50 (0) | 1:10 (−1) | 30 (0) | 24.63 | 5.03 ± <0.01 |
| 21 | 30 (−1) | 50 (0) | 1:20 (1) | 30 (0) | 15.20 | 1.77 ± 0.02 |
| 22 | 60 (1) | 50 (0) | 1:20 (1) | 30 (0) | 25.09 | 4.33 ± 0.02 |
| 23 | 45 (0) | 0 (−1) | 1:15 (0) | 15 (−1) | 14.20 | 2.52 ± 0.01 |
| 24 | 45 (0) | 100 (1) | 1:15 (0) | 15 (−1) | 17.89 | 2.89 ± 0.04 |
| 25 | 45 (0) | 0 (−1) | 1:15 (0) | 45 (1) | 17.60 | 3.18 ± 0.01 |
| 26 | 45 (0) | 100 (1) | 1:15 (0) | 45 (1) | 22.86 | 3.39 ± 0.01 |
| 27 | 45 (0) | 50 (0) | 1:15 (0) | 30 (0) | 17.32 | 2.51 ± 0.01 |
| Source | Global Extraction Yield (YGE) | Phenolic Compounds Yield (YPC) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | Degrees of Freedom | Mean Square | F | p-Value * | Sum of Squares | Degrees of Freedom | Mean Square | F | p-Value * | |
| X1 (L) | 218.16 | 1 | 218.16 | 8034.68 | 0.0001 | 17.87 | 1 | 17.87 | 10,136.20 | 0.0001 |
| X1 (Q) | 5.68 | 1 | 5.68 | 209.04 | 0.0048 | 0.65 | 1 | 0.65 | 366.55 | 0.0027 |
| X2 (L) | 42.91 | 1 | 42.91 | 1580.37 | 0.0010 | 1.93 | 1 | 1.93 | 1096.95 | 0.0009 |
| X2 (Q) | 0.01 | 1 | 0.01 | 0.26 | 0.6596 | 0.75 | 1 | 0.75 | 425.46 | 0.0023 |
| X3 (L) | 4.73 | 1 | 4.73 | 174.26 | 0.0057 | 0.04 | 1 | 0.04 | 20.80 | 0.0449 |
| X3 (Q) | 3.18 | 1 | 3.18 | 117.13 | 0.0084 | 0.95 | 1 | 0.95 | 540.52 | 0.0018 |
| X4 (L) | 91.20 | 1 | 91.20 | 3358.75 | 0.0003 | 3.71 | 1 | 3.71 | 2105.67 | 0.0005 |
| X4 (Q) | 0.32 | 1 | 0.32 | 11.73 | 0.0757 | 0.08 | 1 | 0.08 | 45.54 | 0.0213 |
| X1X2 | 5.65 | 1 | 5.65 | 207.93 | 0.0048 | 0.01 | 1 | 0.01 | 4.14 | 0.1790 |
| X1X3 | 0.13 | 1 | 0.13 | 4.84 | 0.1589 | 0.07 | 1 | 0.07 | 37.97 | 0.0253 |
| X1X4 | 19.41 | 1 | 19.41 | 715.06 | 0.0014 | 0.27 | 1 | 0.27 | 153.97 | 0.0064 |
| X2X3 | 0.11 | 1 | 0.11 | 4.14 | 0.1788 | 0.13 | 1 | 0.13 | 75.33 | 0.0130 |
| X2X4 | 0.63 | 1 | 0.63 | 23.05 | 0.0407 | 0.01 | 1 | 0.01 | 3.81 | 0.1902 |
| X3X4 | <0.001 | 1 | <0.001 | 0.01 | 0.9371 | <0.0001 | 1 | <0.0001 | 0.01 | 0.9493 |
| Lack of fit | 18.07 | 10 | 1.81 | 66.55 | 0.0149 | 1.69 | 10 | 0.17 | 95.73 | 0.0104 |
| Pure Error | 0.05 | 2 | 0.03 | <0.01 | 2 | <0.01 | ||||
| Total | 409.42 | 26 | 27.23 | 26 | ||||||
| R2 | 0.95 | 0.93 | ||||||||
| R2Adj | 0.90 | 0.86 | ||||||||
| Property | UAE 1 | Soxhlet | ||
|---|---|---|---|---|
| Ethanol | Ethanol | n-Hexane | ||
| YGE (wt%) | 28.60 ± 0.80 b | 35.59 ± 0.11 a | 8.57 ± 0.51 c | |
| PC (mg GAE g−1 ORPPF) | 22.29 ± 1.40 b | 30.21 ± <0.01 a | 0.76 ± 0.02 c | |
| FC (mg QE g−1 ORPPF) | 3.93 ± 0.08 b | 5.66 ± 0.15 a | nd | |
| Antioxidant activity (µmol TE g−1 ORPPF) | DPPH• | 103.43 ± 4.89 b | 147.80 ± 6.24 a | 6.61 ± <0.01 c |
| FRAP | 177.37 ± 10.22 b | 217.34 ± 2.98 a | 110.43 ± 0.77 c | |
| Fatty acid (%) 2 | Myristic | 0.48 ± <0.01 b | 1.09 ± 0.07 b | 1.49 ± 0.24 a |
| Palmitic | 16.40 ± 0.24 b | 19.55 ± 0.85 a | 18.59 ± 0.39 a | |
| Stearic | 6.37 ± 0.04 b | 8.75 ± 0.22 a | 8.05 ± 0.21 a | |
| Oleic | 27.19 ± 0.13 b | 28.76 ± 0.10 a | 27.92 ± 0.33 b | |
| Linoleic | 49.55 ± 0.06 a | 42.42 ± 0.43 b | 43.95 ± 0.75 b | |
| Property | UAE 1 | Soxhlet-Ethanol | |
|---|---|---|---|
| Phenolic compound (mg g−1 ORPPF) | Gallic acid | 7.06 ± 0.09 | 6.96 ± 0.09 |
| Trans-Caffeic acid | 0.008 ± <0.001 | 0.009 ± <0.001 | |
| Chlorogenic acid | nd | nd | |
| p-Coumaric acid | 0.01 ± <0.001 | 0.01 ± <0.001 | |
| Vanillic acid | nd | nd | |
| 4-Hydroxybenzoic acid | 0.04 ± 0.001 | 0.04 ± 0.001 | |
| Salicylic acid | nd | nd | |
| Synaptic acid | nd | nd | |
| Syringic acid | 0.08 ± <0.001 | 0.15 ± 0.005 | |
| Trans-Ferrulic acid | 0.02 ± 0.001 | 0.01 ± 0.001 | |
| Quercetin | 0.03 ± 0.001 | 0.08 ± 0.001 | |
| Compound (% normative area) | Elemol | 9.12 | 13.53 |
| γ-Eudesmol | 5.01 | 8.04 | |
| β-Eudesmol | 11.33 | 11.84 | |
| α-Eudesmol | 9.87 | 10.22 | |
| 5-Hydroxymethylfurfural | 11.36 | 10.76 | |
| Bacteria | UAE 1 | Soxhlet-Ethanol | Amoxicillin |
|---|---|---|---|
| Escherichia coli | 62.50 | 125.00 | 3.15 × 10−2 |
| Staphylococcus aureus | 31.25 | 31.25 | 6.30 × 10−2 |
| Pseudomonas aeruginosa | 125.00 | 125.00 | 1.57 × 10−2 |
| Bacillus subtilis | 125.00 | 62.50 | 6.30 × 10−2 |
| Salmonella enterica | 31.25 | 31.25 | 3.15 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mello, A.F.A.; Hoscheid, J.; Raspe, D.T.; Stevanato, N.; da Silva, C. Green Extraction of Oleoresin from Pink Pepper Fruits: Effect of Experimental Conditions and Characterization. AppliedChem 2024, 4, 56-69. https://doi.org/10.3390/appliedchem4010005
de Mello AFA, Hoscheid J, Raspe DT, Stevanato N, da Silva C. Green Extraction of Oleoresin from Pink Pepper Fruits: Effect of Experimental Conditions and Characterization. AppliedChem. 2024; 4(1):56-69. https://doi.org/10.3390/appliedchem4010005
Chicago/Turabian Stylede Mello, Ana Flávia A., Jaqueline Hoscheid, Djéssica T. Raspe, Natália Stevanato, and Camila da Silva. 2024. "Green Extraction of Oleoresin from Pink Pepper Fruits: Effect of Experimental Conditions and Characterization" AppliedChem 4, no. 1: 56-69. https://doi.org/10.3390/appliedchem4010005
APA Stylede Mello, A. F. A., Hoscheid, J., Raspe, D. T., Stevanato, N., & da Silva, C. (2024). Green Extraction of Oleoresin from Pink Pepper Fruits: Effect of Experimental Conditions and Characterization. AppliedChem, 4(1), 56-69. https://doi.org/10.3390/appliedchem4010005