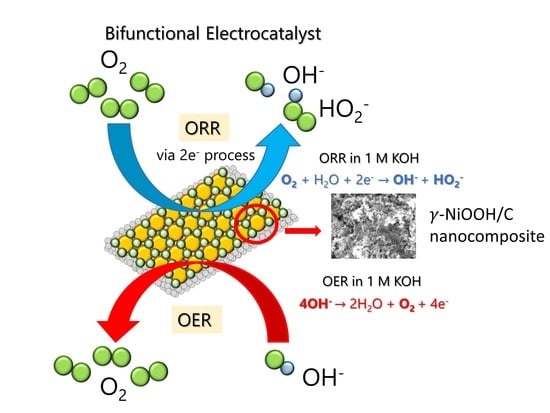
Bifunctional Catalytic Activity of γ-NiOOH toward Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ni(dto) Compound
2.3. Preparation of Working Electrodes
2.4. Electrochemical Performance Investigation
2.5. Pre- and Post-Cycling Electrodes Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Y.; Dong, K.; Yan, X.; Chen, C.; Ni, P.; Yang, C.; Lu, Y. Metal–polydopamine framework-derived (Co)/N-doped carbon hollow nanocubes as efficient oxygen electrocatalysts. Sustain. Energy Fuels 2020, 4, 3370–3377. [Google Scholar] [CrossRef]
- Exner, K.S. Recent progress in the development of screening methods to identify electrode materials for the oxygen evolution reaction. Adv. Funct. Mater. 2020, 30, 2005060. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Tan, P.; Jiang, H.R.; Zhu, X.B.; An, L.; Jung, C.Y.; Wu, M.C.; Shi, L.; Shyy, W.; Zhao, T.S. Advances and challenges in lithium-air batteries. Appl. Energy 2017, 204, 780–806. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Goebel, S.; Greszler, T.; Mathias, M.; Oelerich, W.; Eroglu, D.; Srinivasan, V. Quantifying the promise of lithium–air batteries for electric vehicles. Energy Environ. Sci. 2014, 7, 1555–1563. [Google Scholar] [CrossRef]
- Mainar, A.R.; Leonet, O.; Bengoechea, M.; Boyano, I.; de Meatza, I.; Kvasha, A.; Guerfi, A.; Alberto Blázquez, J. Alkaline aqueous electrolytes for secondary Zinc–air batteries: An overview. Int. J. Energy Res. 2016, 40, 1032–1049. [Google Scholar] [CrossRef]
- Lin, Z.-X.; Lu, Y.-T.; Lai, C.-Y.; Hu, C.-C. Polyvinyl alcohol-based gel electrolytes with high water content for flexible Zinc-air batteries with high rate capability. J. Electrochem. Soc. 2021, 168, 100531. [Google Scholar] [CrossRef]
- Mallick, S.; Samanta, A.; Raj, C.R. Mesoporous carbon-supported manganese tungstate nanostructures for the development of Zinc–air battery powered long lifespan asymmetric supercapacitor. Sustain. Energy Fuels 2020, 4, 4008–4017. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Lin, J.; Shen, Z. Recent advances in air electrodes for Zn–air batteries: Electrocatalysis and structural design. Mater. Horiz. 2017, 4, 945–976. [Google Scholar] [CrossRef]
- Yi, J.; Liu, X.; Liang, P.; Wu, K.; Xu, J.; Liu, Y.; Zhang, J. Non-noble iron group (Fe, Co, Ni)-based oxide electrocatalysts for aqueous Zinc–air batteries: Recent progress, challenges, and perspectives. Organometallics 2019, 38, 1186–1199. [Google Scholar] [CrossRef]
- Yoo, K.; Banerjee, S.; Dutta, P. Modeling of volume change phenomena in a Li–air battery. J. Power Sources 2014, 258, 340–350. [Google Scholar] [CrossRef]
- Li, J.; Yan, F.; Su, Z.; Zhang, T.; Zhang, X.; Sun, H. Highly efficient Li−air battery using linear porosity air electrodes. J. Electrochem. Soc. 2020, 167, 090529. [Google Scholar] [CrossRef]
- Kundu, A.; Mallick, S.; Ghora, S.; Raj, C.R. Advanced oxygen electrocatalyst for air-breathing electrode in Zn-air batteries. ACS Appl. Mater. Interfaces 2021, 13, 40172–40199. [Google Scholar] [CrossRef]
- Yang, W.; Salim, J.; Ma, C.; Ma, Z.; Sun, C.; Li, J.; Chen, L.; Kim, Y. Flowerlike Co3O4 microspheres loaded with copper nanoparticle as an efficient bifunctional catalyst for lithium–air batteries. Electrochem. Commun. 2013, 28, 13–16. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Lin, S.-Y.; Sun, R.-M.; Wang, A.-J.; Zhang, L.; Ma, X.; Feng, J.-J. FeCo/FeCoP encapsulated in N, Mn-codoped three-dimensional fluffy porous carbon nanostructures as highly efficient bifunctional electrocatalyst with multi-components synergistic catalysis for ultra-stable rechargeable Zn-air batteries. J. Colloid Interface Sci. 2022, 605, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Li, X.; Yang, S.; Zu, M.Y.; Liu, P.; Zhang, B.; Zheng, L.R.; Zhao, H.; Yang, H.G. Ni2P(O)/Fe2P(O) interface can boost oxygen evolution electrocatalysis. ACS Energy Lett. 2017, 2, 2257–2263. [Google Scholar] [CrossRef]
- Li, Y.-F.; Selloni, A. Mosaic texture and double c-axis periodicity of β-NiOOH: Insights from first-principles and genetic algorithm calculations. J. Phys. Chem. Lett. 2014, 5, 3981–3985. [Google Scholar] [CrossRef]
- Casas-Cabanas, M.; Canales-Vázquez, J.; Rodríguez-Carvajal, J.; Palacín, M.R. Deciphering the structural transformations during nickel oxyhydroxide electrode operation. J. Am. Chem. Soc. 2007, 129, 5840–5842. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Li, J.-L.; Liu, Z.-P. Structure and catalysis of NiOOH: Recent advances on atomic simulation. J. Phys. Chem. C 2021, 125, 27033–27045. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Z.; Peng, C. Sonochemical intercalation synthesis of nano γ-nickel oxyhydroxide: Structure and electrochemical properties. Electrochim. Acta 2008, 54, 434–441. [Google Scholar] [CrossRef]
- Oliva, P.; Leonardi, J.; Laurent, J.F.; Delmas, C.; Braconnier, J.J.; Figlarz, M.; Fievet, F.; Guibert, A.D. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- Li, L.-F.; Li, Y.-F.; Liu, Z.-P. Oxygen evolution activity on NiOOH catalysts: Four-coordinated Ni cation as the active site and the hydroperoxide mechanism. ACS Catal. 2020, 10, 2581–2590. [Google Scholar] [CrossRef]
- Li, Y.-F.; Selloni, A. Mechanism and activity of water oxidation on selected surfaces of pure and Fe-doped niox. ACS Catal. 2014, 4, 1148–1153. [Google Scholar] [CrossRef]
- Zhao, Z.; Schlexer Lamoureux, P.; Kulkarni, A.; Bajdich, M. Trends in oxygen electrocatalysis of 3 d-layered (oxy)(hydro)oxides. ChemCatChem 2019, 11, 3423–3431. [Google Scholar] [CrossRef]
- Abboudi, M.; Mosset, A. Synthesis of d transition metal sulfides from amorphous dithiooxamide complexes. J. Solid State Chem. 1994, 109, 70–73. [Google Scholar] [CrossRef]
- Abboudi, M.; Mosset, A.; Galy, J. Metal complexes of rubeanic acid. 3. Large-angle x-ray scattering studies of amorphous copper(ii) and nickel(ii) complexes. Inorg. Chem. 1985, 24, 2091–2094. [Google Scholar] [CrossRef]
- Chen, J.G.; Jones, C.W.; Linic, S.; Stamenkovic, V.R. Best practices in pursuit of topics in heterogeneous electrocatalysis. ACS Catal. 2017, 7, 6392–6393. [Google Scholar] [CrossRef] [Green Version]
- Putra, R.P.; Rachman, I.B.; Horino, H.; Rzeznicka, I.I. γ-NiOOH electrocatalyst derived from a nickel dithiooxamide chelate polymer for oxygen evolution reaction in alkaline solutions. Catal. Today 2021, 397, 308–315. [Google Scholar] [CrossRef]
- Mavrič, A.; Fanetti, M.; Lin, Y.; Valant, M.; Cui, C. Spectroelectrochemical tracking of nickel hydroxide reveals its irreversible redox states upon operation at high current density. ACS Catal. 2020, 10, 9451–9457. [Google Scholar] [CrossRef]
- Medway, S.L.; Lucas, C.A.; Kowal, A.; Nichols, R.J.; Johnson, D. In situ studies of the oxidation of nickel electrodes in alkaline solution. J. Electroanal. Chem. 2006, 587, 172–181. [Google Scholar] [CrossRef]
- Seghiouer, A.; Chevalet, J.; Barhoun, A.; Lantelme, F. Electrochemical oxidation of nickel in alkaline solutions: A voltammetric study and modelling. J. Electroanal. Chem. 1998, 442, 113–123. [Google Scholar] [CrossRef]
- Xu, J.; Shi, L.; Wang, J.; Lu, S.; Wang, Y.; Gao, G.; Ding, S. Hierarchical micro/mesoporous nitrogen-doped carbons derived from hypercrosslinked polymers for highly efficient oxygen reduction reaction. Carbon 2018, 138, 348–356. [Google Scholar] [CrossRef]
- Hu, F.; Yang, H.; Wang, C.; Zhang, Y.; Lu, H.; Wang, Q. Co-N-doped mesoporous carbon hollow spheres as highly efficient electrocatalysts for oxygen reduction reaction. Small 2017, 13, 1602507. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- Weast, R.C.; Astle, M.J.; Beyer, W.H. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]
- Li, H.; Liu, H.; Jong, Z.; Qu, W.; Geng, D.; Sun, X.; Wang, H. Nitrogen-doped carbon nanotubes with high activity for oxygen reduction in alkaline media. Int. J. Hydrog. Energy 2011, 36, 2258–2265. [Google Scholar] [CrossRef]
- Erable, B.; Féron, D.; Bergel, A. Microbial catalysis of the oxygen reduction reaction for microbial fuel cells: A review. ChemSusChem 2012, 5, 975–987. [Google Scholar] [CrossRef]
- Fabbri, E.; Mohamed, R.; Levecque, P.; Conrad, O.; Kötz, R.; Schmidt, T.J. Composite electrode boosts the activity of Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite and carbon toward oxygen reduction in alkaline media. ACS Catal. 2014, 4, 1061–1070. [Google Scholar] [CrossRef]
- Huang, J.; Fu, C.; Chen, J.; Senthilkumar, N.; Peng, X.; Wen, Z. The enhancement of selectivity and activity for two-electron oxygen reduction reaction by tuned oxygen defects on amorphous hydroxide catalysts. CCS Chem. 2022, 4, 566–583. [Google Scholar] [CrossRef]
- Xu, H.; Jin, M.; Geng, J.; Zhang, S.; Zhang, H. Bacterial cellulose-regulated synthesis of metallic ni catalysts for high-efficiency electrosynthesis of hydrogen peroxide. Sci. China Mater. 2022, 65, 721–731. [Google Scholar] [CrossRef]
- Trunov, A. Analysis of oxygen reduction reaction pathways on Co3O4, NiCo2o4, Co3O4–Li2O, NiO, NiO–Li2O, Pt, and Au electrodes in alkaline medium. Electrochim. Acta 2013, 105, 506–513. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Alex, C.; John, N.S. Nickel cobalt phosphite nanorods decorated with carbon nanotubes as bifunctional electrocatalysts in alkaline medium with a high yield of hydrogen peroxide. ChemElectroChem 2020, 7, 1935–1942. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Xu, A.-W. Noble-metal-free Fe–N/C catalyst for highly efficient oxygen reduction reaction under both alkaline and acidic conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef]
- Jiang, K.; Back, S.; Akey, A.J.; Xia, C.; Hu, Y.; Liang, W.; Schaak, D.; Stavitski, E.; Nørskov, J.K.; Siahrostami, S.; et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 2019, 10, 3997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Li, H.; Chu, D.; Wang, G. Unraveling oxygen reduction reaction mechanisms on carbon-supported Fe-phthalocyanine and co-phthalocyanine catalysts in alkaline solutions. J. Phys. Chem. C 2009, 113, 20689–20697. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Fan, L.; Han, J.; Xiong, Y. Fe/Ni-N-CNFS electrochemical catalyst for oxygen reduction reaction/oxygen evolution reaction in alkaline media. Appl. Surf. Sci. 2017, 401, 89–99. [Google Scholar] [CrossRef]
- Nadeema, A.; Dhavale, V.M.; Kurungot, S. NiZn double hydroxide nanosheet-anchored nitrogen-doped graphene enriched with the γ-NiOOH phase as an activity modulated water oxidation electrocatalyst. Nanoscale 2017, 9, 12590–12600. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, M.; Yuan, W.; Shi, G. A high-performance three-dimensional Ni–Fe layered double hydroxide/graphene electrode for water oxidation. J. Mater. Chem. 2015, 3, 6921–6928. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, B.; Qin, Z.; Lin, H.; Li, J. Hierarchical wreath-like Au–Co(OH)2 microclusters for water oxidation at neutral pH. Nanoscale 2013, 5, 6826–6833. [Google Scholar] [CrossRef]
- Putra, R.P.; Samejima, Y.; Nakabayashi, S.; Horino, H.; Rzeznicka, I.I. Copper-based electrocatalyst derived from a copper chelate polymer for oxygen reduction reaction in alkaline solutions. Catal. Today 2022, 388–389, 360–364. [Google Scholar] [CrossRef]
- Ding, L.; Xin, Q.; Zhou, X.; Qiao, J.; Li, H.; Wang, H. Electrochemical behavior of nanostructured nickel phthalocyanine (NiPC/C) for oxygen reduction reaction in alkaline media. J. Appl. Electrochem. 2013, 43, 43–51. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Ponraj, J.; Mansour, S.A.; Tarlochan, F. Highly active and stable bi-functional nicoo2 catalyst for oxygen reduction and oxygen evolution reactions in alkaline medium. Int. J. Hydrog. Energy 2019, 44, 16603–16614. [Google Scholar] [CrossRef]
- Ibrahim, K.B.; Su, W.-N.; Tsai, M.-C.; Chala, S.A.; Kahsay, A.W.; Yeh, M.-H.; Chen, H.-M.; Duma, A.D.; Dai, H.; Hwang, B.-J. Robust and conductive magnéli phaseTi4O7 decorated on 3d-nanoflower NiRu-LDH as high-performance oxygen reduction electrocatalyst. Nano Energy 2018, 47, 309–315. [Google Scholar] [CrossRef]
- Qian, L.; Lu, Z.; Xu, T.; Wu, X.; Tian, Y.; Li, Y.; Huo, Z.; Sun, X.; Duan, X. Trinary layered double hydroxides as high-performance bifunctional materials for oxygen electrocatalysis. Adv. Energy Mater. 2015, 5, 1500245. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, J.; Zhang, J. NiCo2S4@graphene as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. ACS Appl. Mater. Interfaces 2013, 5, 5002–5008. [Google Scholar] [CrossRef]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.L.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Cheng, W.; Li, Y.; Zhou, W.; Su, H.; Zhao, X.; Yao, P.; Liu, Q. Metallic Ni3N quantum dots as a synergistic promoter for NiO nanosheet toward efficient oxygen reduction electrocatalysis. J. Phys. Chem. C 2019, 123, 8633–8639. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Q.; Zhao, S.; Wang, S. NiCo2O4 nanoneedle/Mo2C-coated carbon cloth as efficient catalyst for water splitting and metal-air battery. Synth. Met. 2021, 280, 116894. [Google Scholar] [CrossRef]
- Priamushko, T.; Budiyanto, E.; Eshraghi, N.; Weidenthaler, C.; Kahr, J.; Jahn, M.; Tüysüz, H.; Kleitz, F. Incorporation of Cu/Ni in ordered mesoporous co-based spinels to facilitate oxygen evolution and reduction reactions in alkaline media and aprotic Li−O2 batteries. ChemSusChem 2021, 15, e202102404. [Google Scholar] [CrossRef]
- Sancho, H.; Zhang, Y.; Liu, L.; Barevadia, V.G.; Wu, S.; Zhang, Y.; Huang, P.-W.; Zhang, Y.; Wu, T.-H.; You, W.; et al. NiCo2Se4 nanowires as a high-performance bifunctional oxygen electrocatalyst. J. Electrochem. Soc. 2020, 167, 056503. [Google Scholar] [CrossRef]
- Glemser, O.; Einerhand, J. Die struktur Höherer nickelhydroxyde. Z. Anorg. Chem. 1950, 261, 43–51. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, H.; Chen, X.; Liu, H.; Zhao, Y.; Li, H.; Xie, W.; Cheng, F.; Chen, J. Anion insertion enhanced electrodeposition of robust metal hydroxide/oxide electrodes for oxygen evolution. Nat. Commun. 2018, 9, 2373. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Kang, L.; Zhao, T.; Feng, J.; Celorrio, V.; Zhang, G.; Cibin, G.; Kucernak, A.; Brett, D.J.L.; Corà, F.; et al. Identification and manipulation of dynamic active site deficiency-induced competing reactions in electrocatalytic oxidation processes. Energy Environ. Sci. 2022, 15, 2386–2396. [Google Scholar] [CrossRef]
- Liu, Z.X.; Li, Z.P.; Qin, H.Y.; Liu, B.H. Oxygen reduction reaction via the 4-electron transfer pathway on transition metal hydroxides. J. Power Sources 2011, 196, 4972–4979. [Google Scholar] [CrossRef]








| Material | Eonset (V) | Ref. |
|---|---|---|
| NiPc/C | 0.82 | [51] |
| NiO | 0.85 | [52] |
| NiRu-LDH/Ti4O7 | 0.80 | [53] |
| 3D-FL-NiRu-LDH/Ti4O7 | 0.85 | [53] |
| O-NiCoFe-LDH | 0.80 | [54] |
| NiCo2S4@N/S-rGO | 0.85 | [55] |
| cactus-like NiCo2S4@NiFe-LDH | 0.83 | [56] |
| NiCo2S4 | 0.77 | [56] |
| Material | E1/2 (V) | Ref. |
|---|---|---|
| Ni3N QD/NiO heterostructure | 0.76 | [57] |
| Ni3N | 0.69 | [57] |
| NiO | 0.65 | [57] |
| NiCo2O4/Mo2C/CC | 0.79 | [58] |
| NiCo | 0.73 | [59] |
| CuNiCo-2-8 | 0.77 | [59] |
| CuNiCo-8-2 | 0.76 | [59] |
| NiCo2Se4 nanowires | 0.77 | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, R.P.; Rachman, I.B.; Horino, H.; Rzeznicka, I.I. Bifunctional Catalytic Activity of γ-NiOOH toward Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Solutions. Oxygen 2022, 2, 479-492. https://doi.org/10.3390/oxygen2040031
Putra RP, Rachman IB, Horino H, Rzeznicka II. Bifunctional Catalytic Activity of γ-NiOOH toward Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Solutions. Oxygen. 2022; 2(4):479-492. https://doi.org/10.3390/oxygen2040031
Chicago/Turabian StylePutra, Ridwan P., Ihsan Budi Rachman, Hideyuki Horino, and Izabela I. Rzeznicka. 2022. "Bifunctional Catalytic Activity of γ-NiOOH toward Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Solutions" Oxygen 2, no. 4: 479-492. https://doi.org/10.3390/oxygen2040031
APA StylePutra, R. P., Rachman, I. B., Horino, H., & Rzeznicka, I. I. (2022). Bifunctional Catalytic Activity of γ-NiOOH toward Oxygen Reduction and Oxygen Evolution Reactions in Alkaline Solutions. Oxygen, 2(4), 479-492. https://doi.org/10.3390/oxygen2040031