Propagation and Conservation of Horticultural Plants: In Vitro and In Vivo
Share This Topical Collection
Editors
 Dr. Samir C. Debnath
Dr. Samir C. Debnath
 Dr. Samir C. Debnath
Dr. Samir C. Debnath
E-Mail
Website
Collection Editor
Agriculture and Agri-Food Canada, St. John's Research and Development Centre, Newfoundland and Labrador, St. John's, NL A1E 0B2, Canada
Interests: berry crops; breeding; biodiversity; biotechnology; genome editing; epigenetics; in vitro culture; micropropagation; molecular marker; organogenesis; protoplast fusion; somatic embryogenesis; wild germplasm
Special Issues, Collections and Topics in MDPI journals
 Prof. Dr. Shri Mohan Jain
Prof. Dr. Shri Mohan Jain
 Prof. Dr. Shri Mohan Jain
Prof. Dr. Shri Mohan Jain
E-Mail
Website
Collection Editor
Department of Agricultural Sciences, University of Helsinki, PL-27, Helsinki, Finland
Interests: mutation breeding; Haploidy, transgenic crops; genetic diversity; erosion; conservation and utilization; In vitro techniques, molecular- marker-assisted selection and breeding, Digital agriculture, Custom-designed crop breeding
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
In vitro and in vivo propagation is practiced all over the world with horticultural plants. While tubers, rhizomes, slips, stem cuttings, and seeds are mostly used in in vivo propagation, remarkable progress in in vitro culture has resulted in enormous advances in the micropropagation of horticultural crops. Although the automation of bioreactor micropropagation in liquid media has been progressed as a promising way of reducing the cost of propagation, optimal plant propagation depends on a sound understanding of biochemical and physiological responses of the plant to the signals of the culture microenvironment and a standardization of specific chemical and physical culture conditions to optimize the morphogenesis of plant species under a liquid culture system. Plant tissue culture techniques are extensively employed to rapidly multiply true-to-type plants, which provides year-round production. Somaclonal variation, which can be genetic or epigenetic, generally has negative effects on the use of tissue culture propagation. When cultured in an artificial environment, plant cells make numerous genetic alterations and aberrations. The introduction of molecular biology techniques such as DNA-based markers allows the direct comparison of different genetic material, independent of environmental influences. This Special Issue will provide an in-depth look into the progress of in vitro and in vivo propagation along with the use of molecular markers to address fundamental and practical questions of in vitro and in vivo cultures, as well as the employment of molecular markers for the assessment of genetic fidelity, uniformity, stability, and true-to-typeness among donor and micropropagated horticultural plants.
Dr. Samir C. Debnath
Prof. Dr. Shri Mohan Jain
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Agronomy is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- bioreactors
- clonal fidelity
- DNA methylation
- epigenetic variation
- liquid media
- micropropagation
- seed germination
Published Papers (7 papers)
Open AccessEditor’s ChoiceArticle
Development and Optimization of a Rapid In Vitro Micropropagation System for the Perennial Vegetable Night Lily, Hemerocallis citrina Baroni
by
Gaoya Zuo, Ke Li, Yining Guo, Xiaorun Niu, Lijin Yin, Zhiqiang Wu, Xiaomin Zhang, Xiaojing Cheng, Jie Yu, Shaowen Zheng, Yanfang Wang, Guoming Xing, Sen Li and Feifan Hou
Cited by 3 | Viewed by 1645
Abstract
The perennial herbaceous night lily,
Hemerocallis citrina Baroni, is an important vegetable crop with an increasing production and consumption in China. The long lifecycle and slow growth of the night lily are becoming bottlenecks for the large-scale production of elite lines and various
[...] Read more.
The perennial herbaceous night lily,
Hemerocallis citrina Baroni, is an important vegetable crop with an increasing production and consumption in China. The long lifecycle and slow growth of the night lily are becoming bottlenecks for the large-scale production of elite lines and various genetic and breeding studies. There is a lack of a protocol for rapid and efficient micropropagation for this crop. Here, we reported the systematic investigation and optimization of in vitro plant regeneration through tissue-culture-based organogenesis in the night lily variety ‘Datong Huanghua’. We evaluated various factors affecting the efficiency of callus induction and subculture, shoot regeneration, rooting and plantlet establishment, including explant type and age, inoculation methods, basal culture media and the type and concentration of plant growth regulator (phytohormones) in various growth media. We developed an optimized protocol, as follows. The highest efficiency of callus induction was observed on Murashige and Skoog (MS) medium supplied with 22.7 µM TDZ (thidiazuron) using the young scape (flower stem or stalk) as the explant, which was cut longitudinally in half to produce a segment approximately 0.5 cm in length. Callus subculture and proliferation were more efficient on MS medium containing 9.0 µM 2,4-D (2,4-dichlorophenoxyacetic acid) under light culture conditions. Shoot regeneration showed the highest efficiency on MS medium supplemented with 8.9 µM 6-BA (6-benzylaminopurine) + 5.4 µM NAA (α-naphthaleneacetic acid), while the best rooting medium was MS medium containing 2.7 µM NAA. After transplanting, the transplanted regenerated seedlings showed the highest survival rate (96%) on a substrate mixture with a 2:1:1 ratio of peat/perlite/vermiculite. A protocol and flowchart for the rapid in vitro micropropagation of night lily plants is proposed that will facilitate various genetic, genomic and breeding studies on this crop.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceArticle
Evaluation of One-Time Applications of Foliar Applied Auxin Co-Applied with Surfactant for Use in Commercial Cutting Propagation
by
Anthony T. Bowden, Patricia R. Knight, Jenny B. Ryals, Christine E. H. Coker, Scott A. Langlois, Shaun R. Broderick, Eugene K. Blythe, Hamidou F. Sakhanokho and Ebrahiem M. Babiker
Cited by 3 | Viewed by 2406
Abstract
Use of foliar auxin applications are increasing in the nursery and greenhouse industry. However, previous research has shown that insufficient auxin is absorbed or translocated to the site of action when foliar auxin applications are used. It is theorized that adding surfactants to
[...] Read more.
Use of foliar auxin applications are increasing in the nursery and greenhouse industry. However, previous research has shown that insufficient auxin is absorbed or translocated to the site of action when foliar auxin applications are used. It is theorized that adding surfactants to foliar applications of auxin may help with the absorption and translocation of auxin to the site of action. Research was conducted to determine whether adding surfactants to one-time foliar applications of indole-3-butyric acid (IBA) would be as effective as the current industry standard, the basal quick-dip. Terminal, semi-hardwood cuttings of Red Cascade™ miniature climbing rose (
Rosa ‘MOORcap’), common camellia (
Camellia japonica) and ‘Southern Charm’ magnolia (
Magnolia grandiflora ‘Southern Charm’) were sprayed to the drip point using Hortus IBA Water Soluble Salts™ at concentrations of 0 ppm, 50 ppm, 75 ppm, or 100 ppm for rose cuttings or 0 ppm, 500 ppm, 1000 ppm, or 1500 ppm IBA for camellia or magnolia. To serve as an industry control, the basal end of cuttings was immersed for 3-s in a solution of either 250 ppm, 4000 ppm or 2500 ppm for rose, camellia, or magnolia, respectively. A foliar application of 1500 ppm after sticking was as effective as the basal quick-dip for cuttings of ‘Southern Charm’, while other spray treatments were less effective. A basal quick-dip was more effective than a foliar spray for rooting cuttings of camellia. Auxin rate had no impact on rooting of Red Cascade
™ miniature rose. The goal of commercial plant propagation is to produce high-quality rooted cuttings as quickly as possible. Plant propagation places a large demand on labor within the nursery industry, with one recent report being that labor accounts for >50% of a nursery’s budget. Our results from this trial affirm the results reported by similar trials into foliar applications of auxin suggests that the benefits of foliar applications are species dependent Further work is warranted on examining other auxin and surfactant formulations.
Full article
►▼
Show Figures
Open AccessCommunication
Micropropagation of Grapevine and Strawberry from South Russia: Rapid Production and Genetic Uniformity
by
Lavr A. Kryukov, Dmitry I. Vodolazhsky and Rina Kamenetsky-Goldstein
Cited by 11 | Viewed by 5162
Abstract
Grapes and strawberries are major fruit crops with a dynamic, fast-growing global market. In addition to conventional techniques, biotechnological tools allow the preservation of valuable genotypes of both crops and the large-scale propagation of high-quality material. We have developed new protocols for expeditious
[...] Read more.
Grapes and strawberries are major fruit crops with a dynamic, fast-growing global market. In addition to conventional techniques, biotechnological tools allow the preservation of valuable genotypes of both crops and the large-scale propagation of high-quality material. We have developed new protocols for expeditious and reliable micropropagation of grapes and strawberries cultivated in south Russia. In vitro cultivation on semisolid media was combined with rapid propagation in bioreactors. A six-week cycle of propagation in a bioreactor resulted in a 300-fold increase in fresh mass, with a propagation rate of ~5 in grapes and ~20 in strawberries. Genetic analysis using the inter simple sequence repeat (ISSR) DNA fingerprinting technique confirmed the full uniformity of the regenerants. The results of this research will support the local horticultural industry with fast and efficient propagation systems of new breeding lines, introduced varieties, and elite cultivars.
Full article
►▼
Show Figures
Open AccessArticle
The Response of Vegetable Sweet Potato (Ipomoea batatas Lam) Nodes to Different Concentrations of Encapsulation Agent and MS Salts
by
Shehu A. Tadda, Xiaohua Kui, Hongjuan Yang, Min Li, Zhehong Huang, Xuanyang Chen and Dongliang Qiu
Cited by 8 | Viewed by 3878
Abstract
As an emerging technology, shoot encapsulation has been employed in germplasm conservation, distribution, and micropropagation of elite plant species. However, the production of synthetic seeds of sweet potato via non-zygotic embryogenesis requires a large number of embryos per cultured callus suspension and is
[...] Read more.
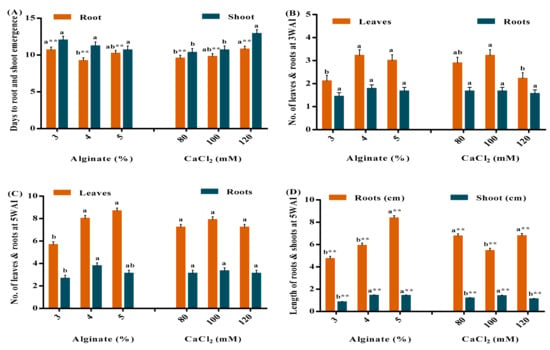
As an emerging technology, shoot encapsulation has been employed in germplasm conservation, distribution, and micropropagation of elite plant species. However, the production of synthetic seeds of sweet potato via non-zygotic embryogenesis requires a large number of embryos per cultured callus suspension and is labour-intensive. Here, we reported a simple method of encapsulating in vitro derived vegetable sweet potato nodal segments with sodium alginate, calcium chloride (CaCl
2), and Murashige and Skoog (MS) salts. The nodes encapsulated with 4% sodium alginate (
w/
v) and 100 mM CaCl
2 were the most suitable for propagation. They had uniform spherical beads and took the least number of days to shoot and root emergence. These plantlets produced more leaves, roots, and long shoots. Further evaluation of the MS salts concentration revealed that the plantlets encapsulated and grown with ½ MS salts had the least days to shoot and root emergence. They also had a longer shoot, the highest conversion rate (99%), and the least leaf abscission (17%). Thus, the sweet potato nodal segments encapsulated with 4% sodium alginate, 100 mM CaCl
2, and ½ MS salts could be used as excellent material for micropropagation, germplasm conservation, and exchange of sweet potato planting materials.
Full article
►▼
Show Figures
Open AccessArticle
Blue Light Upregulates Auxin Signaling and Stimulates Root Formation in Irregular Rooting of Rosemary Cuttings
by
Chan-Saem Gil, Soon-Jae Kwon, Ho-Young Jeong, Chanhui Lee, Oak-Jin Lee and Seok-Hyun Eom
Cited by 10 | Viewed by 3727
Abstract
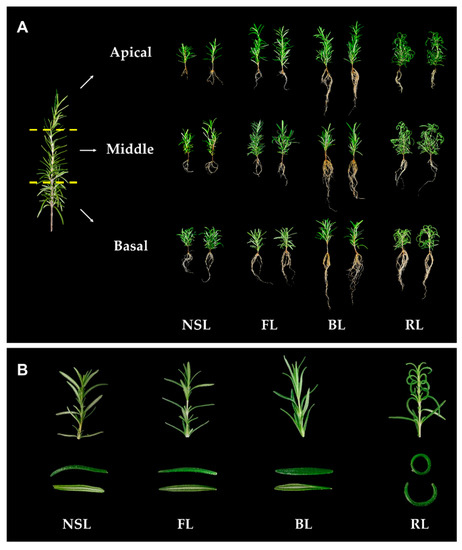
Irregular rooting of rosemary stem cuttings, causing differences in either stem maturation or responses to growth conditions, restricts uniform production. Here, rooting efficiency of apical, middle, and basal cuttings from rosemary stems was evaluated by controlling light conditions to prevent irregular rooting. The
[...] Read more.
Irregular rooting of rosemary stem cuttings, causing differences in either stem maturation or responses to growth conditions, restricts uniform production. Here, rooting efficiency of apical, middle, and basal cuttings from rosemary stems was evaluated by controlling light conditions to prevent irregular rooting. The types of light applied to the cuttings were natural sunlight (NSL), fluorescent, red, and blue (BL) light. Among these light sources, BL significantly induced root growth of not only basal cuttings, but also apical and middle cuttings, whereas NSL induced poor root formation in apical and middle cuttings. In particular, the roots of apical cuttings exposed to BL grew twice as fast as those exposed to other types of light. The overexpression of BL-induced IAA synthetic genes confirmed the rooting patterns. IAA synthetic genes were significantly upregulated by BL in the apical and middle cuttings. Irradiating with 50 μmol photons m
−2 s
−1 BL resulted in similar root production levels among the cutting positions with high biomass, guaranteeing the successful production of uniform cuttings. Thus, the application of proper high-intensity BL promoted healthy, similar-quality rosemary cuttings among stem cutting positions.
Full article
►▼
Show Figures
Open AccessArticle
Optimization of Agrobacterium Mediated Genetic Transformation in Paspalum scrobiculatum L. (Kodo Millet)
by
Ritika Bhatt, Prem Prakash Asopa, Rohit Jain, Aditi Kothari-Chajer, Shanker Lal Kothari and Sumita Kachhwaha
Cited by 14 | Viewed by 3988
Abstract
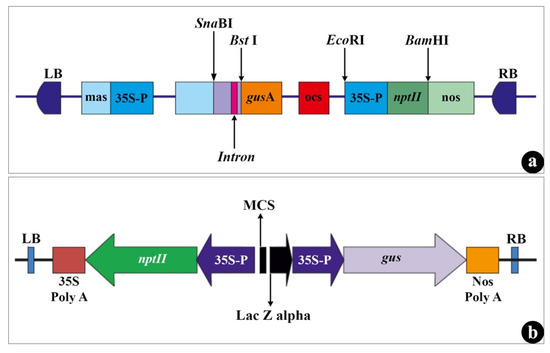
An efficient and reproducible protocol for
Agrobacterium tumefaciens mediated genetic transformation was developed for kodo millet (
Paspalum scrobiculatum L.) by optimizing various parameters.
Agrobacterium strains EHA 105 and LBA 4404 harboring plasmids pCNL 56 and pCAMBIA 2300, respectively, provided the highest transformation
[...] Read more.
An efficient and reproducible protocol for
Agrobacterium tumefaciens mediated genetic transformation was developed for kodo millet (
Paspalum scrobiculatum L.) by optimizing various parameters.
Agrobacterium strains EHA 105 and LBA 4404 harboring plasmids pCNL 56 and pCAMBIA 2300, respectively, provided the highest transformation efficiency. Addition of acetosyringone (AS) in infection medium (200 µM-EHA 105, 250 µM-LBA 4404) and co-cultivation medium (50 µM) increased the transformation efficiency. Transient and stable expression of
gus gene was confirmed with histochemical assay of infected embryos and leaves of transformed plants, respectively. The best GUS response was obtained by pretreatment of callus with an antinecrotic mixture (10 mg/L Cys + 5 mg/L Ag + 2.5 mg/L As) at infection time of 20 min followed by co-cultivation for 3 days (EHA 105) and 5 days (LBA 4404) in dark. Regenerated transgenic plants were obtained after 8 to 10 weeks of selection on callus induction medium (NAA 0.5 mg/L, BAP 1 mg/L) containing 50 mg/L Kan + 250 mg/L Cef and were rooted for 2 weeks on MS medium containing PAA (1 mg/L) and phytagel. The plantlets established in greenhouse showed normal growth. Therefore, the protocol developed in the present study can be used for development of improved varieties of kodo millet.
Full article
►▼
Show Figures
Open AccessArticle
Direct Shoot Organogenesis from Lycium chinense Miller Leaf Explants and Assessment of Genetic Stability Using ISSR Markers
by
Woo-Suk Jung, Ill-Min Chung, Seung-Hyun Kim, Hee-Yeon Chi, Chang Yeon Yu and Bimal Kumar Ghimire
Cited by 13 | Viewed by 3950
Abstract
An efficient in vitro direct shoot regeneration system has been described for
Lycium chinense Miller using leaf explants. Influence of various parameters such as growth regulator concentration, explant type, effect of basal salt type, Murashige and Skoog (1962) medium (MS), Schenk and Hildebrandt
[...] Read more.
An efficient in vitro direct shoot regeneration system has been described for
Lycium chinense Miller using leaf explants. Influence of various parameters such as growth regulator concentration, explant type, effect of basal salt type, Murashige and Skoog (1962) medium (MS), Schenk and Hildebrandt (1972) medium (SH), Gamborg et al. (1968) medium (B5), and carbon sources (sucrose, maltose, and fructose) on the regenerating shoots has been studied. Micromorphological studies and genetic fidelity of regenerated shoots were assessed and compared with those of the donor plants. Among the different concentrations of plant growth regulator (PGRs) tested, MS supplemented with lower concentration of 6-benzylaminopurine (BAP) (0.5 mgL
−1) and thidiazuron (TDZ) (0.5 mgL
−1) increased the frequency of shoot. Comparatively, indole-3-butyric acid (IBA) was more effective in the regeneration and growth of the root system. A higher number of root formation (6.67 ± 1.25) was observed when the rooting medium comprised half-strength MS salts supplemented with 3% sucrose. The surviving plantlets were gradually transferred to the greenhouse and natural soil. More than 90% of the plantlets survived and matured within 85 days. Similarity in the band patterns produced by inter simple sequence repeat (ISSR) primers confirmed the genetic stability and uniformity between the regenerated and donor plants. The present optimized direct shoot regeneration system may be useful for mass propagation and improving the genetic traits in
L. chinense.
Full article
►▼
Show Figures