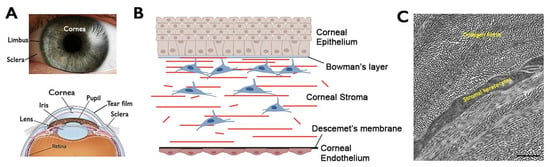
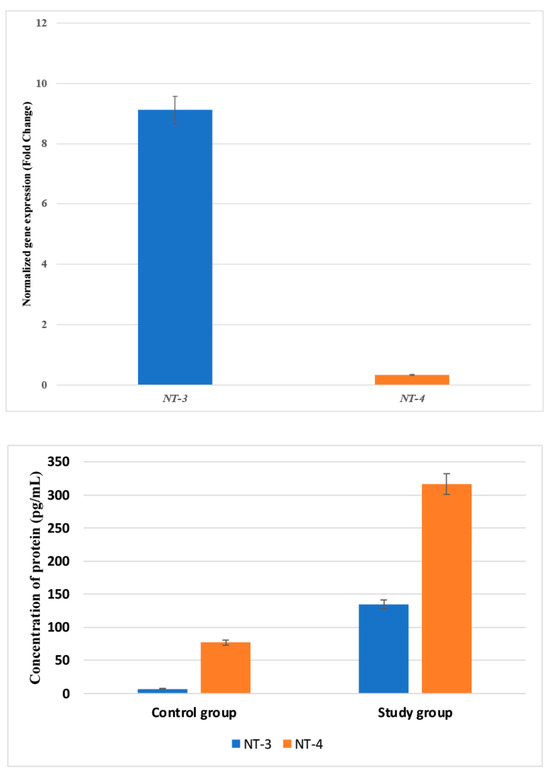
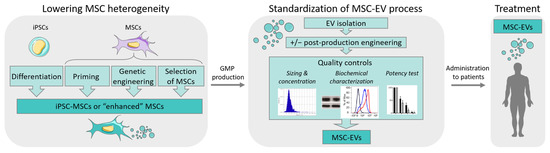
Feature Papers in Gene and Cell Therapy
A topical collection in Biomedicines (ISSN 2227-9059). This collection belongs to the section "Gene and Cell Therapy".
Viewed by 17495Editor
Interests: oligonucleotides-based therapeutics; peptide (cell penetrating peptides)-based delivery vectors; antisense oligonucleotides cellular trafficking
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Recent progresses in gene and cell therapies have been the incentive for this Biomedicine «Feature Papers in Gene and Cell Therapy» Topical Collection. Contributions from the editorial board members, as well as from distinguished scholars in this rapidly growing field will be acknowledged.
Among the recent achievements in the field of gene therapy, CRISPR-directed gene edition has now reached clinical translation, with promising data in the treatment of sickle-cell anemia and thransthyrethin amyloidosis. The great potential of the technology has lead to partnerships between start-up companies and large pharmaceutical companies. Likewise, the successes of mRNA-based vaccines in the SARS-CoV-2 pandemic is giving rise to many developments of mRNA-based platforms to fight human diseases through vaccination or protein-replacement therapies.
Contributions addressing fundamental or clinical researches dealing with nucleic acids-based strategies from oligonucleotides to mRNAs and DNAs, as well as key issues on viral or non-viral delivery vectors will be welcomed.
Cell therapy-based strategies also offer a great potential for the treatment of human diseases. As an example, pluripotent stem cells can be generated from somatic cells with the capacity to proliferate indefinitely and to be reprogrammed for differentiation at will. Challenges dealing with tumorigenicity and immunogenicity have had to be considered, which has delayed the onset of clinical trials until recently.
Contributions dealing with various aspects and applications of cell-based strategies will be welcomed, from animal models to human diseases clinical studies.
Dr. Bernard Lebleu
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Biomedicines is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.