Advances and Future Prospects in Oncolytic Virus Immunotherapy
Share This Topical Collection
Editor
 Dr. Antonio Marchini
Dr. Antonio Marchini
 Dr. Antonio Marchini
Dr. Antonio Marchini
E-Mail
Website
Collection Editor
1. German Cancer Research Center, 69120 Heidelberg, Germany
2. Luxembourg Institute of Health, L-1526 Luxembourg, Luxembourg
Interests: oncolytic viruses; rodent protoparvoviruses; combination therapy; immunogenic cell death; virus-host cell interactions
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Oncolytic viruses (OVs) have attracted special attention due to their ability to self-propagate in the tumou microenvironment and selectively induce the lysis of cancer cells while sparing normal tissues. Growing evidence indicates that OV-mediated cancer cell death is often immunogenic and triggers robust anticancer immune responses and immunoconversion of tumor microenvironments. This makes oncolytic virotherapy a promising new form of immunotherapy and OVs ideal candidates for combination therapy with other anticancer agents, and, in particular, with other immunotherapeutics. There are more than 40 OVs from at least nine different families in clinical development and many more at the preclinical stage. Each OV has its own unique characteristics, mechanisms of action, and advantages to be exploited, but also disadvantages to be mitigated. This collection of Cancers wants to provide a reference point for both basic scientists and clinicians operating in the OV field and, in general, in cancer (immuno)therapy. It will feature original articles describing cutting-edge research in this rapidly evolving field. We also aim to publish authoritative reviews summarizing the state of the art and the new challenges facing oncolytic virus immunotherapy to become a new pillar of cancer treatment.
Dr. Antonio Marchini
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- oncolytic virus
- immunotherapy
- tumour microenvironment
- combination therapy
- personalized virotherapy
- biomarkers
- immunogenic cell death
- virus engineering
Published Papers (6 papers)
Open AccessReview
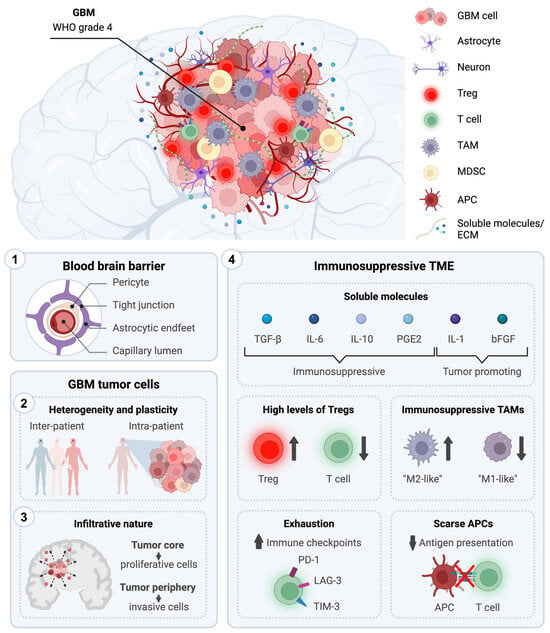
Immunotherapeutic Strategies for the Treatment of Glioblastoma: Current Challenges and Future Perspectives
by
Ilaria Salvato and Antonio Marchini
Cited by 11 | Viewed by 4326
Abstract
Despite decades of research and the best up-to-date treatments, grade 4 Glioblastoma (GBM) remains uniformly fatal with a patient median overall survival of less than 2 years. Recent advances in immunotherapy have reignited interest in utilizing immunological approaches to fight cancer. However, current
[...] Read more.
Despite decades of research and the best up-to-date treatments, grade 4 Glioblastoma (GBM) remains uniformly fatal with a patient median overall survival of less than 2 years. Recent advances in immunotherapy have reignited interest in utilizing immunological approaches to fight cancer. However, current immunotherapies have so far not met the anticipated expectations, achieving modest results in their journey from bench to bedside for the treatment of GBM. Understanding the intrinsic features of GBM is of crucial importance for the development of effective antitumoral strategies to improve patient life expectancy and conditions. In this review, we provide a comprehensive overview of the distinctive characteristics of GBM that significantly influence current conventional therapies and immune-based approaches. Moreover, we present an overview of the immunotherapeutic strategies currently undergoing clinical evaluation for GBM treatment, with a specific emphasis on those advancing to phase 3 clinical studies. These encompass immune checkpoint inhibitors, adoptive T cell therapies, vaccination strategies (i.e., RNA-, DNA-, and peptide-based vaccines), and virus-based approaches. Finally, we explore novel innovative strategies and future prospects in the field of immunotherapy for GBM.
Full article
►▼
Show Figures
Open AccessArticle
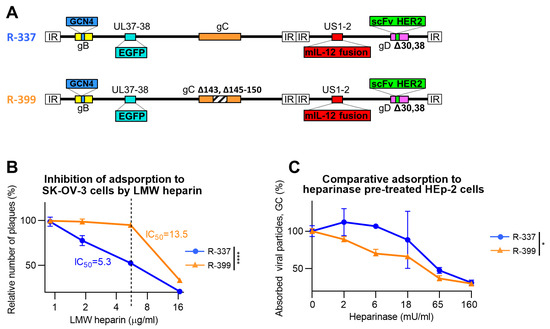
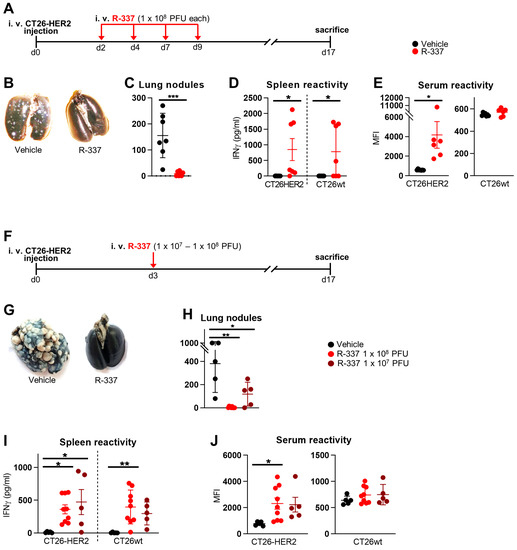
Decrease in Heparan Sulphate Binding in Tropism-Retargeted Oncolytic Herpes Simplex Virus (ReHV) Delays Blood Clearance and Improves Systemic Anticancer Efficacy
by
Andrea Vannini, Federico Parenti, Cristina Forghieri, Gaia Vannini, Catia Barboni, Anna Zaghini, Tatiana Gianni and Gabriella Campadelli-Fiume
Viewed by 1268
Abstract
The role of the interaction with cell-surface glycosaminoglycans (GAGs) during in vivo HSV infection is currently unknown. The rationale of the current investigation was to improve the anticancer efficacy of systemically administered retargeted oHSVs (ReHVs) by decreasing their binding to GAGs, including those
[...] Read more.
The role of the interaction with cell-surface glycosaminoglycans (GAGs) during in vivo HSV infection is currently unknown. The rationale of the current investigation was to improve the anticancer efficacy of systemically administered retargeted oHSVs (ReHVs) by decreasing their binding to GAGs, including those of endothelial cells, blood cells, and off-tumor tissues. As a proof-of-principle approach, we deleted seven amino acids critical for interacting with GAGs from the glycoprotein C (gC) of R-337 ReHV. The modification in the resulting R-399 recombinant prolonged the half-life in the blood of systemically administered R-399 and enhanced its biodistribution to tumor-positive lungs and to the tumor-negative liver. Ultimately, it greatly increased the R-399 efficacy against metastatic-like lung tumors upon IV administration but not against subcutaneous tumors upon IT administration. These results provide evidence that the increased efficacy seen upon R-399 systemic administration correlated with the slower clearance from the circulation. To our knowledge, this is the first in vivo evidence that the partial impairment of the gC interaction with GAGs resulted in a prolonged half-life of circulating ReHV, an increase in the amount of ReHV taken up by tissues and tumors, and, ultimately, an enhanced anticancer efficacy of systemically administered ReHV.
Full article
►▼
Show Figures
Open AccessReview
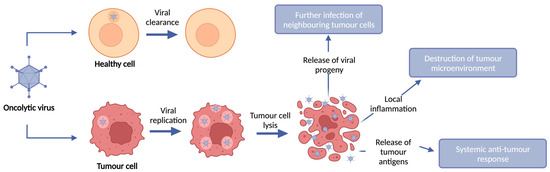
Oncolytic Viruses and Immune Checkpoint Inhibitors: The “Hot” New Power Couple
by
Charlotte Lovatt and Alan L. Parker
Cited by 11 | Viewed by 3545
Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized cancer care and shown remarkable efficacy clinically. This efficacy is, however, limited to subsets of patients with significant infiltration of lymphocytes into the tumour microenvironment. To extend their efficacy to patients who fail to respond or achieve
[...] Read more.
Immune checkpoint inhibitors (ICIs) have revolutionized cancer care and shown remarkable efficacy clinically. This efficacy is, however, limited to subsets of patients with significant infiltration of lymphocytes into the tumour microenvironment. To extend their efficacy to patients who fail to respond or achieve durable responses, it is now becoming evident that complex combinations of immunomodulatory agents may be required to extend efficacy to patients with immunologically “cold” tumours. Oncolytic viruses (OVs) have the capacity to selectively replicate within and kill tumour cells, resulting in the induction of immunogenic cell death and the augmentation of anti-tumour immunity, and have emerged as a promising modality for combination therapy to overcome the limitations seen with ICIs. Pre-clinical and clinical data have demonstrated that OVs can increase immune cell infiltration into the tumour and induce anti-tumour immunity, thus changing a “cold” tumour microenvironment that is commonly associated with poor response to ICIs, to a “hot” microenvironment which can render patients more susceptible to ICIs. Here, we review the major viral vector platforms used in OV clinical trials, their success when used as a monotherapy and when combined with adjuvant ICIs, as well as pre-clinical studies looking at the effectiveness of encoding OVs to deliver ICIs locally to the tumour microenvironment through transgene expression.
Full article
►▼
Show Figures
Open AccessArticle
Efficacy of Systemically Administered Retargeted Oncolytic Herpes Simplex Viruses—Clearance and Biodistribution in Naïve and HSV-Preimmune Mice
by
Andrea Vannini, Federico Parenti, Catia Barboni, Cristina Forghieri, Valerio Leoni, Mara Sanapo, Daniela Bressanin, Anna Zaghini, Gabriella Campadelli-Fiume and Tatiana Gianni
Cited by 2 | Viewed by 1764
Abstract
We investigated the anticancer efficacy, blood clearance, and tissue biodistribution of systemically administered retargeted oncolytic herpes simplex viruses (ReHVs) in HSV-naïve and HSV-preimmunized (HSV-IMM) mice. Efficacy was tested against lung tumors formed upon intravenous administration of cancer cells, a model of metastatic disease,
[...] Read more.
We investigated the anticancer efficacy, blood clearance, and tissue biodistribution of systemically administered retargeted oncolytic herpes simplex viruses (ReHVs) in HSV-naïve and HSV-preimmunized (HSV-IMM) mice. Efficacy was tested against lung tumors formed upon intravenous administration of cancer cells, a model of metastatic disease, and against subcutaneous distant tumors. In naïve mice, HER2- and hPSMA-retargeted viruses, both armed with mIL-12, were highly effective, even when administered to mice with well-developed tumors. Efficacy was higher for combination regimens with immune checkpoint inhibitors. A significant amount of infectious virus persisted in the blood for at least 1 h. Viral genomes, or fragments thereof, persisted in the blood and tissues for days. Remarkably, the only sites of viral replication were the lungs of tumor-positive mice and the subcutaneous tumors. No replication was detected in other tissues, strengthening the evidence of the high cancer specificity of ReHVs, a property that renders ReHVs suitable for systemic administration. In HSV-IMM mice, ReHVs administered at late times failed to exert anticancer efficacy, and the circulating virus was rapidly inactivated. Serum stability and in vivo whole blood stability assays highlighted neutralizing antibodies as the main factor in virus inactivation. Efforts to deplete mice of the neutralizing antibodies are ongoing.
Full article
►▼
Show Figures
Open AccessArticle
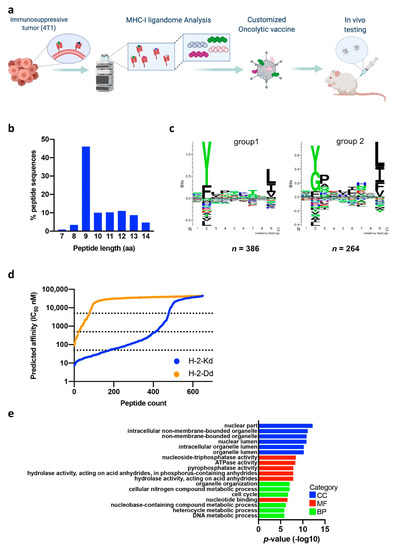
Therapeutic Cancer Vaccination with Immunopeptidomics-Discovered Antigens Confers Protective Antitumor Efficacy
by
Karita Peltonen, Sara Feola, Husen M. Umer, Jacopo Chiaro, Georgios Mermelekas, Erkko Ylösmäki, Sari Pesonen, Rui M. M. Branca, Janne Lehtiö and Vincenzo Cerullo
Cited by 16 | Viewed by 4157
Abstract
Knowledge of clinically targetable tumor antigens is becoming vital for broader design and utility of therapeutic cancer vaccines. This information is obtained reliably by directly interrogating the MHC-I presented peptide ligands, the immunopeptidome, with state-of-the-art mass spectrometry. Our manuscript describes direct identification of
[...] Read more.
Knowledge of clinically targetable tumor antigens is becoming vital for broader design and utility of therapeutic cancer vaccines. This information is obtained reliably by directly interrogating the MHC-I presented peptide ligands, the immunopeptidome, with state-of-the-art mass spectrometry. Our manuscript describes direct identification of novel tumor antigens for an aggressive triple-negative breast cancer model. Immunopeptidome profiling revealed 2481 unique antigens, among them a novel ERV antigen originating from an endogenous retrovirus element. The clinical benefit and tumor control potential of the identified tumor antigens and ERV antigen were studied in a preclinical model using two vaccine platforms and therapeutic settings. Prominent control of established tumors was achieved using an oncolytic adenovirus platform designed for flexible and specific tumor targeting, namely PeptiCRAd. Our study presents a pipeline integrating immunopeptidome analysis-driven antigen discovery with a therapeutic cancer vaccine platform for improved personalized oncolytic immunotherapy.
Full article
►▼
Show Figures
Open AccessArticle
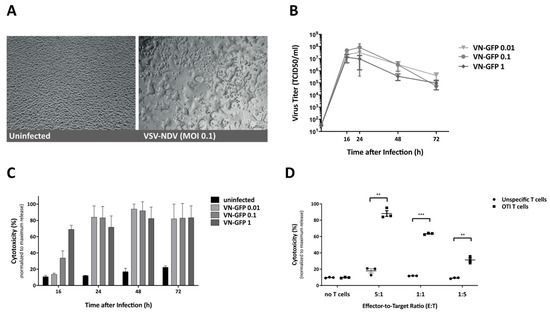
Adoptive T Cell Therapy Is Complemented by Oncolytic Virotherapy with Fusogenic VSV-NDV in Combination Treatment of Murine Melanoma
by
Teresa Krabbe, Janina Marek, Tanja Groll, Katja Steiger, Roland M. Schmid, Angela M. Krackhardt and Jennifer Altomonte
Cited by 11 | Viewed by 3609
Abstract
Cancer immunotherapies have made major advancements in recent years and are becoming the prevalent treatment options for numerous tumor entities. However, substantial response rates have only been observed in specific subsets of patients since pre-existing factors determine the susceptibility of a tumor to
[...] Read more.
Cancer immunotherapies have made major advancements in recent years and are becoming the prevalent treatment options for numerous tumor entities. However, substantial response rates have only been observed in specific subsets of patients since pre-existing factors determine the susceptibility of a tumor to these therapies. The development of approaches that can actively induce an anti-tumor immune response, such as adoptive cell transfer and oncolytic virotherapy, have shown clinical success in the treatment of leukemia and melanoma, respectively. Based on the immune-stimulatory capacity of oncolytic VSV-NDV virotherapy, we envisioned a combination approach to synergize with adoptive T cell transfer, in order to enhance tumor cell killing. Using the immune-competent B16 melanoma model, we demonstrate that combination treatment has beneficial effects on the suppressive microenvironment through upregulation of MHC-I and maintaining low expression levels of PD-L1 on tumor cells. The approach led to additive cytotoxic effects and improved the recruitment of T cells to virus-infected tumor cells in vitro and in vivo. We observed substantial delays in tumor growth and evidence of abscopal effects, as well as prolongation of overall survival time when administered at clinically relevant dosing conditions. Our results indicate that treatment with oncolytic VSV-NDV, combined with adoptive T cell therapy, induces multi-mechanistic and synergistic tumor responses, which supports the further development of this promising translational approach.
Full article
►▼
Show Figures