Targeted Cancer Therapy (Closed)
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Therapy".
Viewed by 10031Editor
Interests: computational cancer genomics; next generation sequencing; targeted therapy; immunotherapy; target discovery; drug repurposing; rare cancers
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
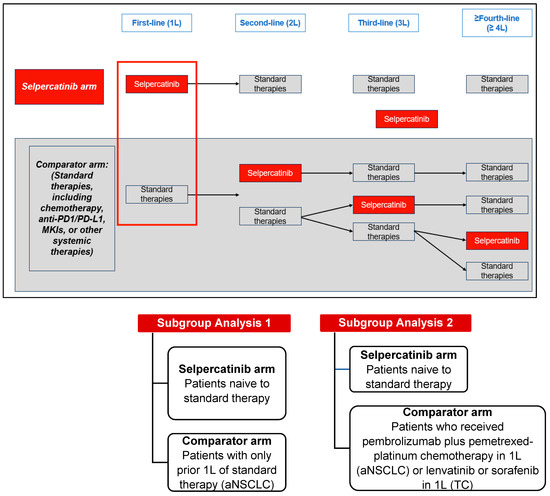
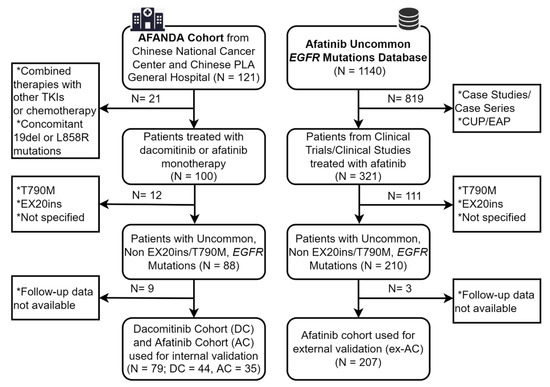
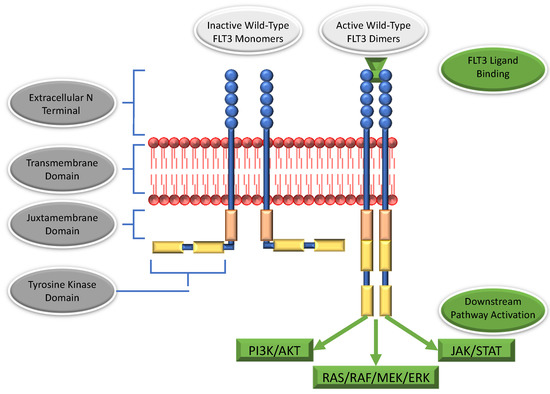
Targeted therapy is one of the major cancer treatment methods available today. The identification of tumor-specific drug targets allows precise targeting of cancer cells while minimizing damage to healthy tissues. Comprehensive genomic characterization of tumor types has helped to advance the development of novel therapies for precision oncology. However, the identification of novel druggable targets, relevant mechanisms, and effective combination therapies is needed. The development of resistance through intrinsic and acquired genomic alterations and signaling to targeted therapy, resulting in exceptional responses lasting for only a short duration, is still a major issue. Furthermore, the success of novel therapies may be hindered by adverse side effects. The objective of the current Topical Collection in Cancers is to publish original research papers and reviews from authors who are interested in addressing these challenges and provide new insights and novel treatment strategies for targeted cancer therapies.
Dr. Jason Roszik
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- targeted therapies
- drug discovery
- combination therapies
- drug resistance mechanisms