HPV-Vaccines
Printed Edition Available!
A printed edition of this Topical Collection is available
here.
Share This Topical Collection
Editor
 Dr. Gloria Calagna
Dr. Gloria Calagna
 Dr. Gloria Calagna
Dr. Gloria Calagna
E-Mail
Website
Collection Editor
“Villa Sofia Cervello” Hospital, University of Palermo, 90133 Palermo, Italy
Interests: gynecology; endoscopy; hysteroscopy; laparoscopy; papillomavirus; colposcopy; HPV vaccines; endometriosis; infertility
Topical Collection Information
Dear Colleague,
Human papillomavirus (HPV) is closely related to the pathogenesis of cervical cancer and many other cancers. HPV vaccines are vaccines that prevent infection with certain types of human papillomavirus. However, despite the various prevention strategies and treatments, such as HPV screening, prophylactic HPV vaccines, surgery, radiotherapy, and chemotherapy, the disease burden remains heavy worldwide. Currently, three types of prophylactic vaccines—quadrivalent HPV vaccines, bivalent HPV vaccines, and a new nonavalent HPV vaccine—are commercially available. Although these vaccines protect against 90% of HPV infections, their capacity to eliminate pre-existing infections is limited.
In this Topical Collection, we aim to systematically cover the progress, current status, and future prospects of various vaccines in development for the prevention and treatment of HPV-associated lesions and cancers.
Dr. Gloria Calagna
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Vaccines is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- HPV
- vaccines
- HPV immunotherapy
- women’s health
Published Papers (17 papers)
Open AccessArticle
The Potential Risk Compensation after Receiving HPV Vaccination among Men Who Have Sex with Men in Southwest China: A HAPA-Based Analysis
by
Zhen Cao, Han Jiang, Wei He, Haiying Pan, Cong Zhang and Xiaoni Zhong
Cited by 1 | Viewed by 1749
Abstract
Background: men who have sex with men (MSM) are a high-risk group for human papillomavirus (HPV) infection, and the HPV vaccine is effective in preventing it. However, according to risk compensation theory, people may increase sexual risk behaviors after receiving HPV vaccination.
[...] Read more.
Background: men who have sex with men (MSM) are a high-risk group for human papillomavirus (HPV) infection, and the HPV vaccine is effective in preventing it. However, according to risk compensation theory, people may increase sexual risk behaviors after receiving HPV vaccination. Based on the Health Action Process Approach (HAPA), this study investigated the influencing factors to predict intention to reduce condom use (risk compensation intention) among MSM after taking HPV vaccination in southwest China.
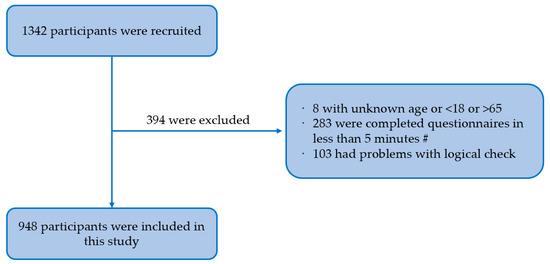
Methods: we conducted a cross-sectional study among 948 MSM in southwest China using a non-probability sampling method and an electronic questionnaire including sociodemographic characteristics, sexual risk behavior characteristics, HPV-related prevention behavior, and the HAPA scale. Confirmatory factor analysis was performed using a structural equation model.
Results: among 948 MSM, the incidence rate of reducing the frequency of condom use was 14.1%. The structural equation model showed that self-efficacy (β = −0.378,
p = 0.020) and positive outcome expectancy (β = 0.366,
p < 0.05) had greater effects on behavioral intention, followed by negative outcome expectancy (β = −0.296,
p < 0.05) and risk perception (β = −0.232,
p < 0.05).
Conclusions: risk compensation may not be a major barrier to receiving HPV vaccination among MSM. Nevertheless, the recognition of possible risk compensation is necessary to implement appropriate interventions to reduce the occurrence of risk compensation.
Full article
►▼
Show Figures
Open AccessReview
Human Papillomavirus Vaccine Impact and Effectiveness in Six High-Risk Populations: A Systematic Literature Review
by
Elizabeth Goodman, Miriam Reuschenbach, Allysen Kaminski and Sarah Ronnebaum
Cited by 11 | Viewed by 4577
|
Correction
Abstract
Specific adult populations known to be at high risk for human papillomavirus (HPV)-related disease, such as men who have sex with men, are inconsistently included in national immunization programs. No compilation of the evidence on the real-world impact and effectiveness of HPV vaccines
[...] Read more.
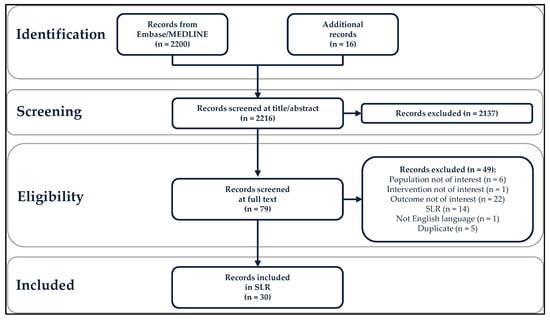
Specific adult populations known to be at high risk for human papillomavirus (HPV)-related disease, such as men who have sex with men, are inconsistently included in national immunization programs. No compilation of the evidence on the real-world impact and effectiveness of HPV vaccines across these populations exists. This systematic literature review identifies and synthesizes the evidence of the real-world impact and effectiveness of the quadrivalent and nonavalent HPV vaccines in high-risk populations: women with prior/current HPV-related anogenital disease, men who have sex with men, immunocompromised/immunosuppressed individuals, female sex workers, transgender and non-binary individuals, and patients with recurrent respiratory papillomatosis (RRP). The outcomes included anogenital precancers/cancers, head and neck cancers, genital warts, and RRP recurrence. From the 2216 records identified, 30 studies (25 effectiveness and 5 impact studies) were included in this systematic literature review. The results, quantity, and quality of these studies were highly variable. The evidence for effectiveness was of high quality only in women with prior/current cervical disease and in individuals with RRP, the most frequently studied populations. No studies of transgender/non-binary individuals or female sex workers were identified. The real-world evidence supports HPV vaccination among women with prior cervical disease and individuals with RRP. Significant real-world data gaps remain in these high-risk populations.
Full article
►▼
Show Figures
Open AccessCase Report
Juvenile Membranous Nephropathy Developed after Human Papillomavirus (HPV) Vaccination
by
Haruna Arakawa, Shohei Yokoyama, Takehiro Ohira, Dedong Kang, Kazuho Honda, Yoshihiko Ueda and Akihiro Tojo
Cited by 1 | Viewed by 3194
Abstract
A 16-year-old girl with no history of renal disease had a fever of 38 °C after her second HPV vaccination and was identified as positive for proteinuria. As she maintained urinary protein of 3.10 g/gCr and 5–9 urinary red blood cells/HPF, a renal
[...] Read more.
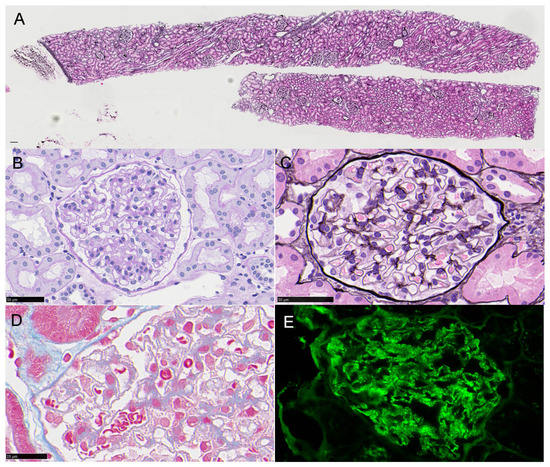
A 16-year-old girl with no history of renal disease had a fever of 38 °C after her second HPV vaccination and was identified as positive for proteinuria. As she maintained urinary protein of 3.10 g/gCr and 5–9 urinary red blood cells/HPF, a renal biopsy was performed and small spikes on PAM staining with the granular deposition of IgG1++ and IgG3+ on the glomerular capillary wall were discovered by immunofluorescence, although PLA2R immunostaining was negative. Analysis by electron microscope showed electron density deposition in the form of fine particles under the epithelium. The diagnosis was secondary membranous nephropathy stage II. Immunostaining with the anti-p16 INK4a antibody was positive for glomerular cells, and Western blot analysis of urinary protein showed a positive band for p16 INK4a. However, laser-microdissection mass spectrometry analysis of a paraffin section of glomeruli failed to detect HPV proteins. It is possible that the patient was already infected with HPV and administration of the HPV vaccine may have caused secondary membranous nephropathy.
Full article
►▼
Show Figures
Open AccessArticle
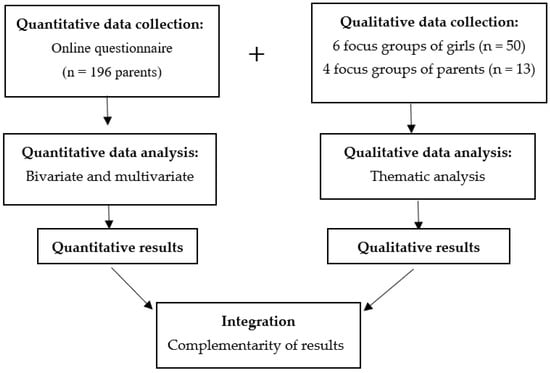
HPV-Vaccine Hesitancy in Colombia: A Mixed-Methods Study
by
Veronica Cordoba-Sanchez, Mariantonia Lemos, Diego Alfredo Tamayo-Lopera and Sherri Sheinfeld Gorin
Cited by 6 | Viewed by 4492
Abstract
In Colombia, the uptake rate of the HPV vaccine dropped from 96.7% after its introduction in 2013 to 9% in 2020. To identify the behavioural components of HPV-vaccine hesitancy in females aged 15 and under and their families, we conducted a convergent mixed-methods
[...] Read more.
In Colombia, the uptake rate of the HPV vaccine dropped from 96.7% after its introduction in 2013 to 9% in 2020. To identify the behavioural components of HPV-vaccine hesitancy in females aged 15 and under and their families, we conducted a convergent mixed-methods study in which 196 parents/caregivers responded to an online questionnaire and 10 focus groups were held with 13 of these parents/caregivers, and 50 age-eligible girls. The study is novel as it is the first to explore the factors influencing HPV-vaccine hesitancy alongside the COVID vaccine within an integrative model of behaviour change, the capability-opportunity-motivation-behaviour (COM-B) model. We found that COVID-19 has had an impact on the awareness of HPV and HPV vaccination. Lack of information about the vaccination programs, concerns about vaccine safety and the relationship between HPV and sexuality could be related to vaccine hesitancy. Trust in medical recommendations and campaigns focused on the idea that vaccination is a way of protecting daughters from cervical cancer could improve HPV vaccine uptake.
Full article
►▼
Show Figures
Open AccessArticle
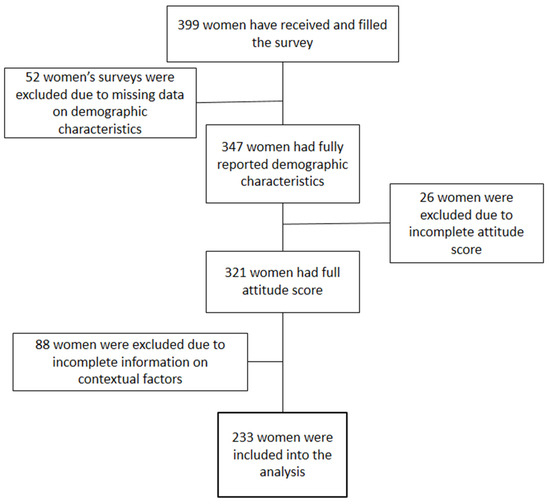
What Factors Are Associated with Attitudes towards HPV Vaccination among Kazakhstani Women? Exploratory Analysis of Cross-Sectional Survey Data
by
Gulzhanat Aimagambetova, Aisha Babi, Torgyn Issa and Alpamys Issanov
Cited by 23 | Viewed by 4064
Abstract
Background. The high prevalence of HPV infection among Kazakhstani women and the absence of an HPV vaccination program are directly reflected in increasing rates of cervical cancer incidence and mortality. Kazakhstan made its first attempt at introducing the HPV vaccine in 2013, but
[...] Read more.
Background. The high prevalence of HPV infection among Kazakhstani women and the absence of an HPV vaccination program are directly reflected in increasing rates of cervical cancer incidence and mortality. Kazakhstan made its first attempt at introducing the HPV vaccine in 2013, but was unsuccessful due to complications and low public acceptance. The attitudes of Kazakhstani women towards the vaccine were never measured. Therefore, this study aims to investigate the attitudes of women towards the HPV vaccine and determine factors associated with positive, negative, or neutral attitudes.
Methods. A 29-item survey consisting of 21 demographic and contextual questions and 8 Likert-scale questions was distributed among women attending gynecological offices in four major cities of Kazakhstan from December 2021 until February 2022. Attitudes of women were measured based on their answers to the eight Likert-scale questions. Ordinal logistic regression was built to find associations between demographic characteristics and attitudes of women.
Results. Two hundred thirty-three women were included in the final analysis. A total of 54% of women had positive attitudes towards the vaccine. The majority of women did not trust or had a neutral attitude towards the government, pharmaceutical industry, and traditional and alternative media. However, the trust of women was high in medical workers and scientific researchers. Women’s age, education, number of children, effect of the 2013 HPV program, and trust in alternative medicine were included in the ordinal logistic model. Women with a low level of education, a high number of children, who believe in alternative medicine, and who were affected by the failed 2013 vaccination program were less likely to have a positive attitude towards the vaccine.
Conclusions. Contrary attitudes towards HPV vaccination exist among Kazakhstani women, with approximately half having positive and almost half having negative or neutral attitudes towards the vaccine. An informational campaign that takes into consideration women’s levels of trust in different agencies, as well as targets those who are the most uninformed, might help in a successful relaunch of the HPV vaccination program. However, more studies that cover a higher number of women are required.
Full article
►▼
Show Figures
Open AccessArticle
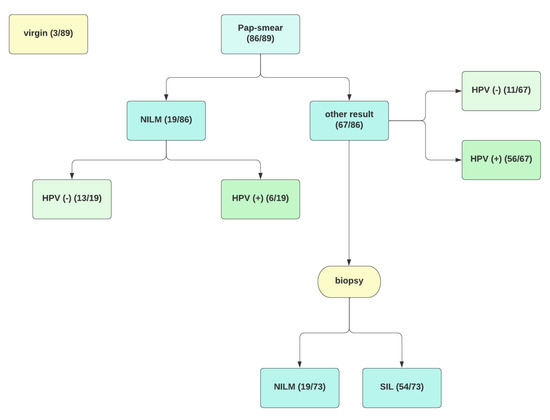
Immunity after HPV Vaccination in Patients after Sexual Initiation
by
Dominik Pruski, Małgorzata Łagiedo-Żelazowska, Sonja Millert-Kalińska, Jan Sikora, Robert Jach and Marcin Przybylski
Cited by 3 | Viewed by 4578
Abstract
Vaccinations against human papillomavirus (HPV) are included in the primary prevention of precancerous intraepithelial lesions and HPV-related cancers. Despite the undeniable effectiveness of vaccination in the juvenile population, there is still little research on the effect in patients after sexual initiation. Our study
[...] Read more.
Vaccinations against human papillomavirus (HPV) are included in the primary prevention of precancerous intraepithelial lesions and HPV-related cancers. Despite the undeniable effectiveness of vaccination in the juvenile population, there is still little research on the effect in patients after sexual initiation. Our study aims to assess anti-HPV (L1 HPV) antibodies in healthy patients and diagnosed cervical pathology after 9-valent vaccination. We provide a prospective, ongoing 12-month, non-randomised pilot study in which 89 subjects were enrolled. We used an enzyme-linked immunosorbent assay to determine IgG class antibodies to HPV. We noted significantly higher levels of antibodies in vaccinated individuals than in the unvaccinated control group. The above work shows that vaccination against HPV might be beneficial in patients after sexual initiation as well as in those already diagnosed with HPV or SIL infection.
Full article
►▼
Show Figures
Open AccessArticle
Adherence to the Recommended HPV Vaccine Dosing Schedule among Adolescents Aged 13 to 17 Years: Findings from the National Immunization Survey-Teen, 2019–2020
by
Chinenye Lynette Ejezie, Ikponmwosa Osaghae, Sylvia Ayieko and Paula Cuccaro
Cited by 7 | Viewed by 3058
Abstract
The 9-valent human papillomavirus (9-vHPV) vaccine uptake rate among adolescents has improved over the years; however, little is known about the adherence to the recommended dosing schedule. This study examines the prevalence and factors associated with adherence to the recommended 9vHPV vaccination dosing
[...] Read more.
The 9-valent human papillomavirus (9-vHPV) vaccine uptake rate among adolescents has improved over the years; however, little is known about the adherence to the recommended dosing schedule. This study examines the prevalence and factors associated with adherence to the recommended 9vHPV vaccination dosing schedule among adolescents aged 13 to 17 years. The cross-sectional study was conducted using the 2019–2020 National Immunization Survey-Teen. The parents of 34,619 adolescents were included in our analyses. The overall up-to-date (UTD) prevalence was 57.1%. The UTD prevalence was 60.0% among females and 54.2% among males. Adolescents aged 16 years had the highest UTD prevalence of 63.0%. The UTD prevalence was 61.6% among Hispanics and 54.7% among non-Hispanic Whites. Overall, compared to females, males had 14% lower odds of UTD. The odds of UTD were 1.91 times, 2.08 times, and 1.98 times higher among adolescents aged 15–17 years, respectively, compared to those aged 13 years. Moreover, region, poverty, insurance status, mothers’ educational level, and provider recommendation were associated with UTD. Our findings show that adherence to the recommended 9vHPV vaccine schedule is low in the US. Targeted public health efforts are needed to improve the rates of adherence to the recommended 9vHPV dose schedule.
Full article
Open AccessArticle
Real-Life Safety Profile of the 9-Valent HPV Vaccine Based on Data from the Puglia Region of Southern Italy
by
Antonio Di Lorenzo, Paola Berardi, Andrea Martinelli, Francesco Paolo Bianchi, Silvio Tafuri and Pasquale Stefanizzi
Cited by 4 | Viewed by 3069
Abstract
Human Papillomavirus (HPV) is responsible for epithelial lesions and cancers in both males and females. The latest licensed HPV vaccine is Gardasil-9
®, a 9-valent HPV vaccine which is effective not only against the high-risk HPV types, but also against the ones
[...] Read more.
Human Papillomavirus (HPV) is responsible for epithelial lesions and cancers in both males and females. The latest licensed HPV vaccine is Gardasil-9
®, a 9-valent HPV vaccine which is effective not only against the high-risk HPV types, but also against the ones responsible for non-cancerous lesions. This report describes adverse events following Gardasil-9
® administration reported in Puglia, southern Italy, from January 2018 to November 2021. This is a retrospective observational study. Data about the adverse events following immunization (AEFIs) with Gardasil-9
® were collected from the Italian Drug Authority database. AEFIs were classified as serious or non-serious accordingly to World Health Organization guidelines, and serious ones underwent causality assessment. During the study period, 266,647 doses of 9vHPVv were administered in Puglia and 22 AEFIs were reported, with a reporting rate (RR) of 8.25 per 100,000 doses. The most reported symptoms were neurological ones (7/22). A total of 5 (22.7%) AEFIs were classified as serious, and 2 of these led to the patient’s hospitalization. In one case, permanent impairment occurred. Following causality assessment, only 2 out of 5 serious AEFIs were deemed to be consistently associated with the vaccination (RR: 0.750 per 100,000 doses). The data gathered in our study are similar to the pre-licensure evidence as far as the nature of the AEFIs is concerned. The reporting rate, though, is far lower than the ones described in clinical trials, likely due to the different approach to data collection: in our study, data were gathered via passive surveillance, while pre-marketing studies generally employ active calls for this purpose. Gardasil-9
®’s safety profile appears to be favorable, with a low rate of serious adverse events and a risk/benefits ratio pending for the latter.
Full article
Open AccessArticle
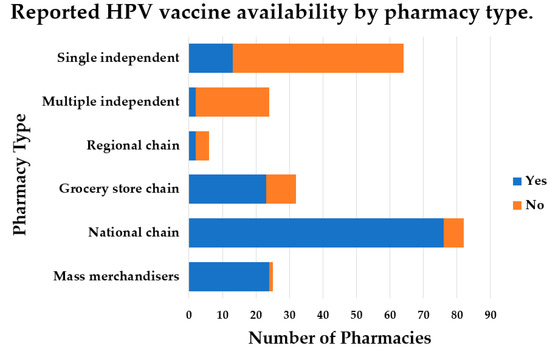
Availability of the HPV Vaccine in Regional Pharmacies and Provider Perceptions Regarding HPV Vaccination in the Pharmacy Setting
by
Jill M. Maples, Nikki B. Zite, Oluwafemifola Oyedeji, Shauntá M. Chamberlin, Alicia M. Mastronardi, Samantha Gregory, Justin D. Gatwood, Kenneth C. Hohmeier, Mary E. Booker, Jamie D. Perry, Heather K. Moss and Larry C. Kilgore
Cited by 3 | Viewed by 3202
Abstract
There is increasing support for HPV vaccination in the pharmacy setting, but the availability of the HPV vaccine is not well known. Additionally, little is known about perceptions of medical providers regarding referring patients to community pharmacies for HPV vaccination. The purpose of
[...] Read more.
There is increasing support for HPV vaccination in the pharmacy setting, but the availability of the HPV vaccine is not well known. Additionally, little is known about perceptions of medical providers regarding referring patients to community pharmacies for HPV vaccination. The purpose of this study was to determine HPV vaccine availability in community pharmacies and to understand, among family medicine and obstetrics–gynecology providers, the willingness of and perceived barriers to referring patients for HPV vaccination in a pharmacy setting. HPV vaccine availability data were collected from pharmacies in a southern region of the United States. Family medicine and obstetrics–gynecology providers were surveyed regarding vaccine referral practices and perceived barriers to HPV vaccination in a community pharmacy. Results indicated the HPV vaccine was available in most pharmacies. Providers were willing to refer patients to a community pharmacy for HPV vaccination, despite this not being a common practice, likely due to numerous barriers reported. Pharmacist-administered HPV vaccination continues to be a commonly reported strategy for increasing HPV vaccination coverage. However, coordinated efforts to increase collaboration among vaccinators in different settings and to overcome systematic and legislative barriers to increasing HPV vaccination rates are still needed.
Full article
►▼
Show Figures
Open AccessArticle
Parents’ Knowledge and Attitude towards HPV and HPV Vaccination in Poland
by
Katarzyna Smolarczyk, Anna Duszewska, Slawomir Drozd and Slawomir Majewski
Cited by 26 | Viewed by 4646
Abstract
HPV is one of the diseases of civilization that causes cervical cancer, among other diseases. For this reason, a vaccination program has been introduced worldwide for preadolescent, sexually inactive seronegative girls. However, the decision to vaccinate young girls must be made by the
[...] Read more.
HPV is one of the diseases of civilization that causes cervical cancer, among other diseases. For this reason, a vaccination program has been introduced worldwide for preadolescent, sexually inactive seronegative girls. However, the decision to vaccinate young girls must be made by the parents. In Poland, vaccinations are recommended but not financed by the government, which affects their choices, and there is insufficient knowledge of the diseases caused by genital HPV types. In addition, there are cultural, social, and even religious factors to be considered. Therefore, the aim of the study was to analyze the state of knowledge about HPV and HPV vaccines among parents. Two hundred and eighty-eight parents participated in the study, but only 180 of them declared that they had ever heard of HPV (62.5%). Therefore, only these parents completed the entire questionnaire consisting of 34 questions. The parents’ answers were analyzed with the Fisher’s and chi-squared tests. The study showed that parents’ knowledge of HPV and HPV vaccination in Poland is low (49.4% of correct answers). Parents’ attitudes were only influenced by knowledge and education and not by other parameters such as age, gender, place of residence, and the number of children. This study indicates that parents need to be educated about the threats of HPV and the possibilities of prophylactic vaccination.
Full article
Open AccessReview
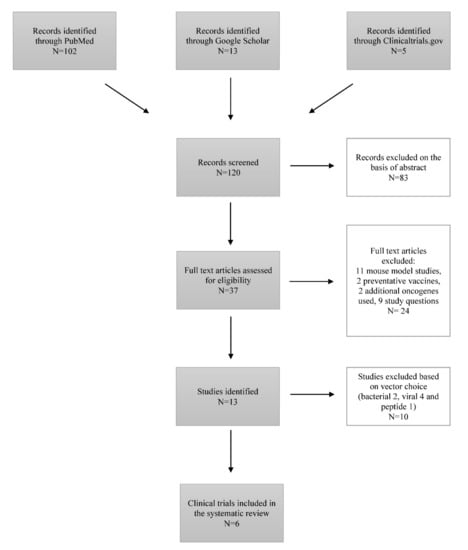
The Efficacy of Therapeutic DNA Vaccines Expressing the Human Papillomavirus E6 and E7 Oncoproteins for Treatment of Cervical Cancer: Systematic Review
by
Ayazhan Akhatova, Chee Kai Chan, Azliyati Azizan and Gulzhanat Aimagambetova
Cited by 21 | Viewed by 5183
Abstract
Cervical cancer is recognized as a serious public health problem since it remains one of the most common cancers with a high mortality rate among women despite existing preventative, screening, and treatment approaches. Since Human Papillomavirus (HPV) was recognized as the causative agent,
[...] Read more.
Cervical cancer is recognized as a serious public health problem since it remains one of the most common cancers with a high mortality rate among women despite existing preventative, screening, and treatment approaches. Since Human Papillomavirus (HPV) was recognized as the causative agent, the preventative HPV vaccines have made great progress over the last few years. However, people already infected with the virus require an effective treatment that would ensure long-term survival and a cure. Currently, clinical trials investigating HPV therapeutic vaccines show a promising vaccine-induced T-cell mediated immune response, resulting in cervical lesion regression and viral eradication. Among existing vaccine types (live vector, protein-based, nucleic acid-based, etc.), deoxyribonucleic acid (DNA) therapeutic vaccines are the focus of the study, since they are safe, cost-efficient, thermostable, easily produced in high purity and distributed. The aim of this study is to assess and compare existing DNA therapeutic vaccines in phase I and II trials, expressing HPV E6 and E7 oncoproteins for the prospective treatment of cervical cancer based on clinical efficacy, immunogenicity, viral clearance, and side effects. Five different DNA therapeutic vaccines (GX-188E, VGX-3100, pNGVL4a-CRT/E7(detox), pNGVL4a-Sig/E7(detox)/HSP70, MEDI0457) were well-tolerated and clinically effective. Clinical implementation of DNA therapeutic vaccines into treatment regimen as a sole approach or in combination with conservative treatment holds great potential for effective cancer treatment.
Full article
►▼
Show Figures
Open AccessSystematic Review
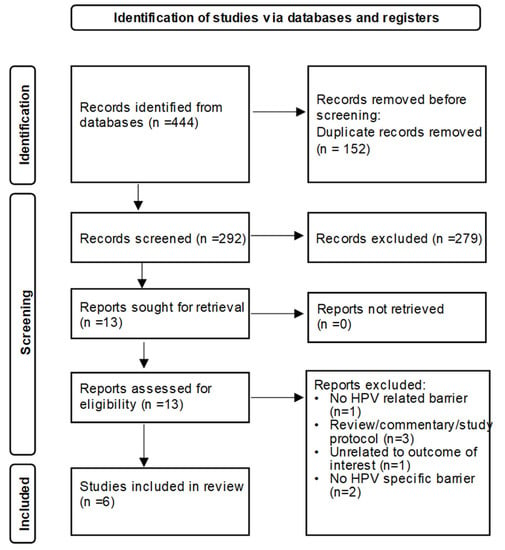
Pharmacists’ Perceived Barriers to Human Papillomavirus (HPV) Vaccination: A Systematic Literature Review
by
Oluwafemifola Oyedeji, Jill M. Maples, Samantha Gregory, Shauntá M. Chamberlin, Justin D. Gatwood, Alexandria Q. Wilson, Nikki B. Zite and Larry C. Kilgore
Cited by 11 | Viewed by 4984
Abstract
About 45:000 cancers are linked to HPV each year in the United States alone. The HPV vaccine prevents cancer and is highly effective, yet vaccination coverage remains low. Pharmacies can play a meaningful role in increasing HPV vaccination access due to their availability
[...] Read more.
About 45:000 cancers are linked to HPV each year in the United States alone. The HPV vaccine prevents cancer and is highly effective, yet vaccination coverage remains low. Pharmacies can play a meaningful role in increasing HPV vaccination access due to their availability and convenience. However, little is known about pharmacists’ perceived barriers to HPV vaccination. The objective of this systematic review was to summarize existing literature on perceived barriers to administering HPV vaccination reported by pharmacists. Barriers identified from selected studies were synthesized and further grouped into patient, parental, (pharmacist’s) personal, and system/organization barrier groups. Six studies were included in this review. The cost of the HPV vaccine, insurance coverage and reimbursement were commonly reported perceived barriers. Adolescent HPV vaccination barriers related to parental concerns, beliefs, and inadequate knowledge about the HPV vaccine. Perceived (pharmacist’s) personal barriers were related to lack of information and knowledge about HPV vaccine and recommendations. At the system/organization level, barriers reported included lack of time/staff/space; difficulty in series completion; tracking and recall of patient; perceived competition with providers; and other responsibilities/vaccines taking precedence. Future strategies involving pharmacy settings in HPV-related cancer prevention efforts should consider research on multilevel pharmacy-driven interventions addressing barriers.
Full article
►▼
Show Figures
Open AccessArticle
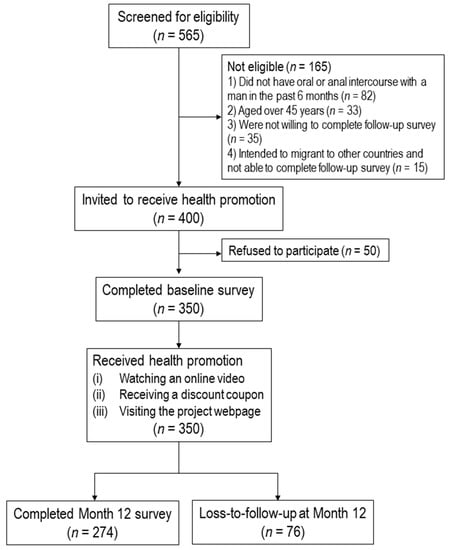
Effectiveness of a Community-Based Organization—Private Clinic Service Model in Promoting Human Papillomavirus Vaccination among Chinese Men Who Have Sex with Men
by
Zixin Wang, Yuan Fang, Paul Shing-fong Chan, Andrew Chidgey, Francois Fong, Mary Ip and Joseph T. F. Lau
Cited by 10 | Viewed by 2920
Abstract
This study evaluated the effectiveness of the community-based organization (CBO)-private clinic service model in increasing human papillomavirus (HPV) vaccination uptake among unvaccinated men who have sex with men (MSM) in Hong Kong during a 12-month follow-up period. A CBO-private clinic model was implemented
[...] Read more.
This study evaluated the effectiveness of the community-based organization (CBO)-private clinic service model in increasing human papillomavirus (HPV) vaccination uptake among unvaccinated men who have sex with men (MSM) in Hong Kong during a 12-month follow-up period. A CBO-private clinic model was implemented to promote HPV vaccination among Chinese MSM. A CBO with good access to MSM approached MSM aged 18–45 years who had never received an HPV vaccination, invited them to receive an online health promotion, and referred them to receive HPV vaccination at gay-friendly private clinics. A baseline survey and a follow-up evaluation at Month 12 were conducted. A total of 350 participants completed the baseline survey. Among 274 participants who were followed up at Month 12, 46 (16.8%) had taken up at least one dose of HPV vaccination. After adjusting for significant baseline characteristics, the perceived susceptibility (AOR:1.25,
p = 0.002) and perceived severity (AOR:1.21,
p = 0.003) of HPV and HPV-related diseases, perceived benefits (AOR:1.16,
p = 0.03), self-efficacy to receive HPV vaccination (AOR:1.37,
p = 0.001), and behavioral intention to take up HPV vaccination at baseline (AOR:6.99,
p < 0.001) significantly predicted HPV vaccination uptake. The process evaluation of the program was positive. The CBO-private clinic service model was helpful in increasing HPV vaccination uptake among MSM.
Full article
►▼
Show Figures
Open AccessArticle
A Long-Term Observation on the Possible Adverse Effects in Japanese Adolescent Girls after Human Papillomavirus Vaccination
by
Akiyo Hineno and Shu-Ichi Ikeda
Cited by 4 | Viewed by 5298
Abstract
In Japan, a significant number of adolescent females noted unusual symptoms after receiving the human papillomavirus (HPV) vaccination, of which the vast majority of them were initially diagnosed with psychiatric illnesses because of the absence of pathologic radiological images and specific abnormalities in
[...] Read more.
In Japan, a significant number of adolescent females noted unusual symptoms after receiving the human papillomavirus (HPV) vaccination, of which the vast majority of them were initially diagnosed with psychiatric illnesses because of the absence of pathologic radiological images and specific abnormalities in laboratory test results. Later these symptoms were thought to be adverse effects of HPV vaccination. However, a causal link between HPV vaccination and the development of these symptoms has not been demonstrated. Between June 2013 and March 2021, we examined 200 patients who noted various symptoms after HPV vaccination. In total, 87 were diagnosed with HPV vaccination-related symptoms based on our proposed diagnostic criteria. The clinical histories of these 87 patients were analyzed. The age at initial vaccination ranged from 11 to 19 years old (mean ± SD: 13.5 ± 1.5 years old), and the age at the first appearance of symptoms ranged from 12 to 20 years old (mean ± SD: 14.3 ± 1.6 years old). The patients received an initial HPV vaccine injection between May 2010 and May 2013, but the first affected patient developed symptoms in October 2010, and the last affected developed symptoms in October 2015. A cluster of patients with a post-HPV vaccination disorder has not appeared in Japan during the last five years. Our study shows that, in Japan, the period of HPV vaccination considerably overlapped with that of a unique post-HPV vaccination disorder development. This disorder appears as a combination of orthostatic intolerance, chronic regional pain syndrome, and cognitive dysfunction, but its exact pathogenesis remains unclear.
Full article
►▼
Show Figures
Open AccessReview
A Systematic Review of Interventions to Improve HPV Vaccination Coverage
by
Edison J. Mavundza, Chinwe J. Iwu-Jaja, Alison B. Wiyeh, Blessings Gausi, Leila H. Abdullahi, Gregory Halle-Ekane and Charles S. Wiysonge
Cited by 48 | Viewed by 7421
Abstract
Human papillomavirus (HPV) infection is the most common sexually transmitted infection worldwide. Although most HPV infections are transient and asymptomatic, persistent infection with high-risk HPV types may results in diseases. Although there are currently three effective and safe prophylactic HPV vaccines that are
[...] Read more.
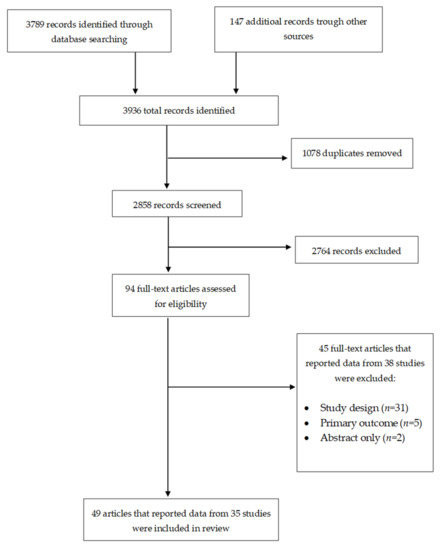
Human papillomavirus (HPV) infection is the most common sexually transmitted infection worldwide. Although most HPV infections are transient and asymptomatic, persistent infection with high-risk HPV types may results in diseases. Although there are currently three effective and safe prophylactic HPV vaccines that are used across the world, HPV vaccination coverage remains low. This review evaluates the effects of the interventions to improve HPV vaccination coverage. We searched the Cochrane Central Register of Controlled Trials, PubMed, Web of Science, Scopus, and the World Health Organization International Clinical Trials Registry Platform and checked the reference lists of relevant articles for eligible studies. Thirty-five studies met inclusion criteria. Our review found that various evaluated interventions have improved HPV vaccination coverage, including narrative education, outreach plus reminders, reminders, financial incentives plus reminders, brief motivational behavioral interventions, provider prompts, training, training plus assessment and feedback, consultation, funding, and multicomponent interventions. However, the evaluation of these intervention was conducted in high-income countries, mainly the United States of America. There is, therefore, a need for studies to evaluate the effect of these interventions in low-and middle-income countries, where there is a high burden of HPV and limited HPV vaccination programs.
Full article
►▼
Show Figures
Open AccessReview
Internal and External Validity of Social Media and Mobile Technology-Driven HPV Vaccination Interventions: Systematic Review Using the Reach, Effectiveness, Adoption, Implementation, Maintenance (RE-AIM) Framework
by
Matthew Asare, Braden Popelsky, Emmanuel Akowuah, Beth A. Lanning and Jane R. Montealegre
Cited by 12 | Viewed by 4103
Abstract
Social media human papillomavirus (HPV) vaccination interventions show promise for increasing HPV vaccination rates. An important consideration for the implementation of effective interventions into real-world practice is the translation potential, or external validity, of the intervention. To this end, we conducted a systematic
[...] Read more.
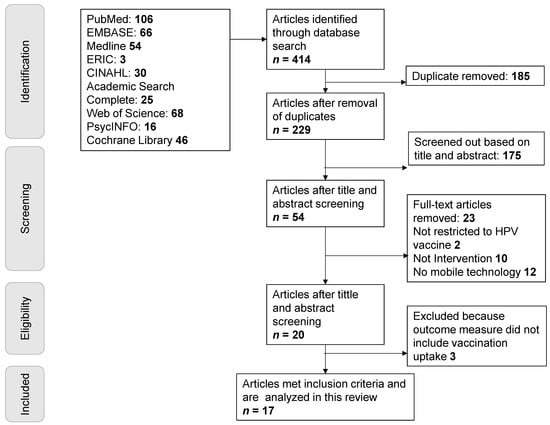
Social media human papillomavirus (HPV) vaccination interventions show promise for increasing HPV vaccination rates. An important consideration for the implementation of effective interventions into real-world practice is the translation potential, or external validity, of the intervention. To this end, we conducted a systematic literature review to describe the current body of evidence regarding the external validity of social media HPV vaccination-related interventions. Constructs related to external validity were based on the reach, effectiveness, adoption, implementation, maintenance (RE-AIM) framework. Seventeen articles published between 2006 and 2020 met the inclusion criteria. Three researchers independently coded each article using a validated RE-AIM framework. Discrepant codes were discussed with a fourth reviewer to gain consensus. Of these 17 studies, 3 were pilot efficacy studies, 10 were randomized controlled trials (RCTs) to evaluate effectiveness, 1 was a population-based study, and 3 did not explicitly state which type of study was conducted. Reflecting this distribution of study types, across all studies the mean level of reporting RE-AIM dimensions varied with reach recording 90.8%, effectiveness (72.1%), adoption (40.3%), implementation (45.6%), and maintenance (26.5%). This review suggests that while the current HPV vaccination social media-driven interventions provide sufficient information on internal validity (reach and effectiveness), few have aimed to gather data on external validity needed to translate the interventions into real world implementation. Our data suggest that implementation research is needed to move HPV vaccination-related interventions into practice. Included in this review are recommendations for enhancing the design and reporting of these HPV vaccination social media-related interventions.
Full article
►▼
Show Figures