Effect of the Postural Challenge on the Dependence of the Cardiovascular Control Complexity on Age
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Experimental Protocol
2.3. Data Acquisition
2.4. Time Series Extraction
2.5. Complexity Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Population
3.2. Time Domain Parameters: Aging and Gender Effects
3.3. NCIHP and NCISAP at REST: Aging and Gender Effects
3.4. NCIHP and NCISAP during STAND: Aging and Gender Effects
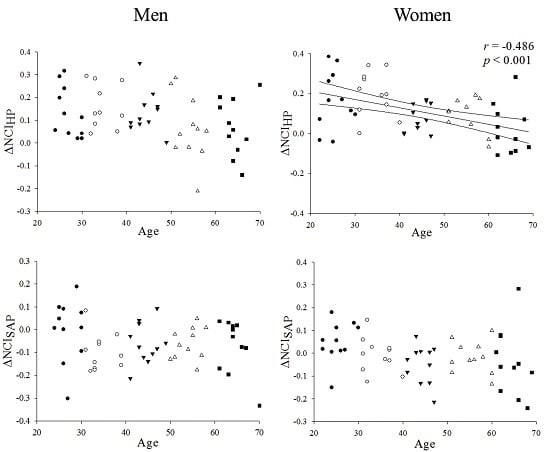
3.5. ΔNCIHP and ΔNCISAP: Aging and Gender Effects
3.6. Linear Correlation Analysis between NCI and Time Domain Parameters
4. Discussion
4.1. Selection of the Population
4.2. Complexity of the HP Variability at REST: Aging and Gender Effects
4.3. Complexity of the SAP Variability at REST: Aging and Gender Effects
4.4. Complexity of HP and SAP Variabilities during STAND: Aging and Gender Effects
4.5. Individual Changes in the Complexity of HP and SAP Variabilities in Response to STAND
4.6. Linear Regression Analysis of NCI on Time Domain Parameters
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goldberger, A.L.; Peng, C.K.; Lipsitz, L.A. What is physiologic complexity and how does it change with aging and disease? Neurobiol. Aging 2002, 23, 23–26. [Google Scholar]
- Pikkujämsä, S.M.; Mäkikallio, T.H.; Sourander, L.B.; Räihä, I.J.; Puukka, P.; Skyttä, J.; Peng, C.K.; Goldberger, A.L.; Huikuri, H.V. Cardiac interbeat interval dynamics from childhood to senescence: Comparison of conventional and new measures based on fractals and chaos theory. Circulation 1999, 100, 393–399. [Google Scholar]
- Lipsitz, L.A.; Mietus, J.; Moody, G.B.; Goldberger, A.L. Spectral characteristics of heart rate variability before and during postural tilt: Relations to aging and risk of syncope. Circulation 1990, 81, 1803–1810. [Google Scholar]
- Kaplan, D.T.; Furman, M.; Pincus, S.M.; Ryan, S.M.; Lipsitz, L.A.; Goldberger, A.L. Aging and the complexity of cardiovascular dynamics. Biophys. J. 1991, 59, 945–949. [Google Scholar]
- Bigger, J.T., Jr.; Fleiss, J.L.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992, 85, 164–171. [Google Scholar]
- Catai, A.M.; Chacon-Mikahil, M.P.; Martinelli, F.S.; Forti, V.A.; Silva, E.; Golfetti, R.; Martins, L.E.; Szrajer, J.S.; Wanderley, J.S.; Lima-Filho, E.C.; et al. Effects of aerobic exercise training on heart rate variability during wakefulness and sleep and cardiorespiratory responses of young and middle-aged healthy men. Braz. J. Med. Biol. Res. 2002, 35, 741–752. [Google Scholar]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar]
- Melo, R.C.; Santos, M.D.; Silva, E.; Quiterio, R.J.; Moreno, M.A.; Reis, M.S.; Verzola, I.A.; Oliveira, L.; Martins, L.E.; Gallo, L., Jr.; et al. Effects of age and physical activity on the autonomic control of heart rate in healthy men. Braz. J. Med. Biol. Res. 2005, 38, 1331–1338. [Google Scholar]
- Zhang, J. Effect of age and sex on heart rate variability in healthy subjects. J. Manip. Physiol. Ther. 2007, 30, 374–379. [Google Scholar]
- Mancia, G.; Ferrari, A.; Gregorini, L.; Parati, G.; Pomidossi, G.; Bertinieri, G.; Grassi, G.; di Rienzo, M.; Pedotti, A.; Zanchetti, A. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ. Res. 1983, 53, 96–104. [Google Scholar]
- Mann, S.; Millar Craig, M.W.; Altman, D.G.; Raftery, E.B.; Hunyor, S.N. Blood pressure variability in health, hypertension and autonomic failure. Clin. Exp. Hypertens. 1985, 7, 187–194. [Google Scholar]
- Watson, R.D.S.; Stallard, T.J.; Flinn, R.M.; Littler, W.A. Factors determining direct arterial pressure and its variability in hypertensive man. Hypertension 1980, 2, 333–341. [Google Scholar]
- Beckers, F.; Verheyden, B.; Aubert, A.E. Aging and nonlinear heart rate control in a healthy population. Am. J. Physiol. 2006, 290, H2560–H2570. [Google Scholar]
- Viola, A.U.; Tobaldini, E.; Chellappa, S.L.; Rabello Casali, K.; Porta, A.; Montano, N. Short-term complexity of cardiac autonomic control during sleep: REM as a potential risk factor for cardiovascular system in aging. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Takahashi, A.C.M.; Porta, A.; Melo, R.C.; Quitério, R.J.; da Silva, E.; Borghi-Silva, A.; Tobaldini, E.; Montano, N.; Catai, A.M. Aging reduces complexity of heart rate variability by conditional entropy and symbolic analysis. Intern. Emerg. Med. 2012, 7, 229–235. [Google Scholar]
- Vandeput, S.; Verheyden, B.; Aubert, A.E.; van Huffel, S. Nonlinear heart rate dynamics: Circadian profile and influence of age and gender. Med. Eng. Phys. 2012, 34, 108–117. [Google Scholar]
- Nemati, S.; Edwards, B.A.; Lee, J.; Pittman-Polletta, B.; Butler, J.P.; Malhotra, A. Respiration and heart rate complexity: Effects of age and gender assessed by band-limited transfer entropy. Respir. Physiol. Neurobiol. 2013, 189, 27–33. [Google Scholar]
- Porta, A.; Faes, L.; Bari, V.; Marchi, A.; Bassani, T.; Nollo, G.; Perseguini, N.M.; Milan, J.; Minatel, V.; Borghi-Silva, A.; et al. Effect of age on complexity and causality of the cardiovascular control: Comparison between model-based and model-free approaches. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Mueller, A.; Bonnemeier, H.; Malberg, H.; Kurths, J.; Wessel, N. Age-Dependent Changes in the Manifestations of gender-related differences in the cardiovascular regulation. Proceedings of the 8th Conference of the European Study Group on Cardiovascular Oscillations (ESGCO 2014), Trento, Italy, 25–28 May 2014; pp. 147–148.
- Perseguini, N.M.; Takahashi, A.C.M.; Rebelatto, J.R.; Silva, E.; Borghi-Silva, A.; Porta, A.; Montano, N.; Catai, A.M. Spectral and symbolic analysis of the effect of gender and postural change on cardiac autonomic modulation in health elderly subjects. Braz. J. Med. Biol. Res. 2011, 44, 29–37. [Google Scholar]
- Ryan, S.M.; Goldberger, A.L.; Pincus, S.M.; Mietus, J.; Lipsitz, L.A. Gender- and age-related differences in heart rate dynamics: are women more complex than men? J. Am. Coll. Card. 1994, 24, 1700–1707. [Google Scholar]
- Voss, A.; Heitmann, A.; Schroeder, R.; Peters, A.; Perz, S. Short-term heart rate variability—Age dependence in healthy subjects. Physiol. Meas. 2012, 33, 1289–1311. [Google Scholar]
- Voss, A.; Schroeder, R.; Fisher, C.; Heitmann, A.; Peters, A.; Perz, S. Influence of age and gender on complexity measures for short-term heart rate variability analysis in healthy subjects. Proceedings of the 35th Annual International Conference of the IEEE EMBS, Osaka, Japan, 3–7 July 2013; pp. 5574–5577.
- Porta, A.; Baselli, G.; Liberati, D.; Montano, N.; Cogliati, C.; Gnecchi-Ruscone, T.; Malliani, A.; Cerutti, S. Measuring regularity by means of a corrected conditional entropy in sympathetic outflow. Biol. Cybern. 1998, 78, 71–78. [Google Scholar]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Magagnin, V.; Bassani, T.; Bari, V.; Turiel, M.; Maestri, R.; Pinna, G.D.; Porta, A. Non-stationarities significantly distort short-term spectral, symbolic and entropy heart rate variability indexes. Physiol. Meas. 2011, 32, 1775–1786. [Google Scholar]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. 2000, 278, H2039–H2049. [Google Scholar]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. 1991, 88, 2297–2301. [Google Scholar]
- Porta, A.; Faes, L.; Masé, M.; D’Addio, G.; Pinna, G.D.; Maestri, R.; Montano, N.; Furlan, R.; Guzzetti, S.; Nollo, G.; et al. An integrated approach based on uniform quantization for the evaluation of complexity of short-term heart period variability: Application to 24 h Holter recordings in healthy and heart failure humans. Chaos 2007, 17. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Casaburi, R.; Whipp, B.J. Principles of Exercise Testing and Interpretation, 3rd ed; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1999. [Google Scholar]
- Fleg, J.L.; Strait, J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Fail. Rev. 2012, 17, 545–554. [Google Scholar]
- Burtscher, M. Exercise limitations by the oxygen delivery and utilization systems in aging and disease: Coordinated adaptation and deadaptation of the lung-heart muscle axis—A mini-review. Gerontology 2013, 59, 289–296. [Google Scholar]
- Dubnov, G.; Brzezinski, A.; Berry, M. Weight control and the management of obesity after menopause: The role of physical activity. Maturitas 2003, 44, 89–101. [Google Scholar]
- Lipsitz, L.A.; Goldberger, A.L. Loss of “complexity” and aging: potential applications of fractals and chaos theory to senescence. J. Am. Med. Assoc. 1992, 267, 1806–1809. [Google Scholar]
- Porta, A.; Gnecchi-Ruscone, T.; Tobaldini, E.; Guzzetti, S.; Furlan, R.; Montano, N. Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J. Appl. Physiol. 2007, 103, 1143–1149. [Google Scholar]
- Convertino, V.A. Gender differences in autonomic functions associated with blood pressure regulation. Am. J. Physiol. 1998, 275, R1909–R1920. [Google Scholar]
- Laitinen, T.; Hartikainen, J.; Vanninen, E.; Niskanen, L.; Geelen, G.; Lansimies, E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J. Appl. Physiol. 1998, 84, 576–583. [Google Scholar]
- Kuo, T.B.; Lin, T.; Yang, C.C.; Li, C.L.; Chen, C.F.; Chou, P. Effect of aging on gender differences in neural control of heart rate. Am J. Physiol. 1999, 277, H2233–H2239. [Google Scholar]
- Evans, J.M.; Ziegler, M.G.; Patwardhan, A.R.; Ott, J.B.; Kim, C.S.; Leonelli, F.M.; Knapp, C.F. Gender differences in autonomic cardiovascular regulation: Spectral, hormonal and hemodynamic indexes. J. Appl. Physiol. 2001, 91, 2611–2618. [Google Scholar]
- Pikkujämsä, S.M.; Mäkikallio, T.H.; Airaksinen, K.E.J.; Huikuri, H.V. Determinants and interindividual variation of R-R interval dynamic in healthy middle-aged subjects. Am. J. Physiol. 2001, 280, H1400–H1406. [Google Scholar]
- Ramaekers, D.; Ector, H.; Aubert, A.E.; Rubens, A.; Van de Werf, F. Heart rate variability in healthy volunteers. Is the female autonomic system cardioprotective? Eur. Heart J. 1998, 19, 1334–1341. [Google Scholar]
- Bonnemeier, H.; Wiegand, U.K.H.; Brandes, A.; Kluge, N.; Katus, H.A.; Richardt, G.; Potratz, J. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: Differing effects of aging and gender on heart rate variability. J. Cardiovasc. Electrophysiol. 2003, 14, 791–799. [Google Scholar]
- Porta, A.; Castiglioni, P.; di Rienzo, M.; Bari, V.; Bassani, T.; Marchi, A.; Takahashi, A.C.M.; Tobaldini, E.; Montano, N.; Catai, A.M.; et al. Short-term complexity indexes of heart period and systolic arterial pressure variabilities provide complementary information. J. Appl. Physiol. 2012, 113, 1810–1820. [Google Scholar]
- Mendelshon, M.E. Protective effects of estrogen on the cardiovascular system. Am. J. Cardiol. 2002, 89, 12E–17E. [Google Scholar]
- Mercuro, G.; Podda, A.; Pitzalis, L.; Zoncu, S.; Mascia, M.; Melis, G.B.; Rosano, G.M. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am. J. Cardiol. 2000, 85, 787–789. [Google Scholar]
- Hayward, C.S.; Kelly, R.P.; Collins, P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc. Res. 2000, 46, 28–49. [Google Scholar]
- Genazzani, A.R. Controversial issues in climacteric medicine (I) Cardiovascular disease and hormone replacement therapy. In Maturitas, International Menopause Society Expert Workshop, royal society of medicine, London, UK, 13–16 October 2000; 2001; 38, pp. 263–271. [Google Scholar]
- Laitinen, T.; Niskamen, L.; Geelen, G.; Lansimies, E.; Hartikainen, J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J. Appl. Physiol. 2004, 96, 2333–2340. [Google Scholar]
- Kikuya, M.; Hozawa, A.; Ohokubo, T.; Tsuji, I.; Michimata, M.; Matsubara, M.; Ota, M.; Nagai, K.; Araki, T.; Satoh, H.; et al. Prognostic significance of blood pressure and heart rate variabilities: The Ohasama study. Hypertension 2000, 36, 901–906. [Google Scholar]
- Singh, D.; Vinod, K.; Saxena, S.C.; Deepa, K.K. Spectral evaluation of aging effects on blood pressure and heart rate variations in healthy subjects. J. Med. Eng. Techn. 2006, 30, 145–150. [Google Scholar]
- Stauss, H.M. Identification of blood pressure control mechanisms by power spectral analysis. Clin. Exp. Pharmacol. Physiol. 2007, 34, 362–368. [Google Scholar]
- Lakatta, E.G. Arterial aging is risky. J. Appl. Physiol. 2008, 105, 1321–1322. [Google Scholar]
- Liu, C.C.; Kuo, T.B.J.; Yang, C.C.H. Effects of estrogen on gender-related autonomic differences in humans. Am. J. Physiol. 2003, 285, H2188–H2193. [Google Scholar]
- Rosano, G.M.C.; Patrizi, R.; Leonardo, F.; Ponikowski, P.; Collins, P.; Sarrel, P.M.; Chierchia, S.L. Effect of estrogen replacement therapy on heart rate variability and heart rate in healthy postmenopausal women. Am. J. Cardiol. 1997, 80, 815–817. [Google Scholar]
- Turianikova, Z.; Javorka, K.; Baumert, M.; Calkovska, A.; Javorka, M. The effect of orthostatic stress on multiscale entropy of heart rate and blood pressure. Physiol. Meas. 2011, 32, 1425–1437. [Google Scholar]
- Baselli, G.; Porta, A.; Pagani, M. Coupling arterial windkessel with peripheral vasomotion: Modeling the effects on low-frequency oscillations. IEEE Trans. Biomed. Eng. 2006, 53, 53–64. [Google Scholar]
- Barnett, S.R.; Morin, R.J.; Kiely, D.K.; Gagnon, M.; Azhar, G.; Knight, E.L.; Nelson, J.C.; Lipsitz, L.A. Effects of age and gender on autonomic control of blood pressure dynamics. Hypertension 1999, 33, 1195–1200. [Google Scholar]
- Veermann, D.P.; Imholz, B.P.M.; Wieling, W.; Karemaker, J.M.; van Mortfrans, G.A. Effects of aging on blood pressure variability in resting conditions. Hypertension 1994, 24, 120–130. [Google Scholar]
- Zulfiqar, U.; Jurivich, D.A.; Gao, W.; Singer, D.H. Relation of high heart rate variability to healthy longevity. Am. J. Cardiol. 2010, 105, 1181–1185. [Google Scholar]
- Jones, P.P.; Christou, D.D.; Jordan, J.; Seals, D.R. Baroreflex buffering is reduced with age in healthy men. Circulation 2003, 107, 1770–1774. [Google Scholar]





| Age bin | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 |
|---|---|---|---|---|---|
| 110 volunteers | 11 M/11 F | 11 M/11 F | 11 M/11 F | 11 M/11 F | 11 M/11 F |
| Age (years) | 26 (24–29) | 33 (32–37) | 44 (43–46) | 55 (51–57) | 64 (62–66) |
| Height (cm) | 169 (164–178) | 168 (163–174) | 170 (160–174) | 168 (159–172) | 163 (155–167) |
| Weight (kg) | 67.5 (59.5–73.0) | 65.1 (60.0–75.0) | 73.0 (64.1–83.0) | 65.2 (58.0–73.0) | 67.5 (62.0–73.0) |
| BMI (kg/m2) | 23 (21–24) | 24 (22–25) | 26 (23–28)*§ | 25 (23–25) | 25 (24–27)* |
| VO2 peak (mL/kg/min) | 34 (28–40) | 36 (28–43) | 29 (24–38) | 27 (23–34)§ | 24 (19–30)§ |
| Men | 11 M | 11 M | 11 M | 11 M | 11 M |
| Age (years) | 26 (25–30) | 33 (32–37) | 44 (43–47) | 55 (51–56) | 64 (63–66) |
| Height (cm) | 174 (170–182) | 172 (168–178) | 174 (170–181) | 171 (168–177) | 166 (164–176) |
| Weight (kg) | 73.0 (68.6–80.0) | 75.0 (69.1–78.2) | 80.0 (76.1–86.7) | 72.0 (69.0–80.9) | 68.2 (65.5–76.6) |
| BMI (kg/m2) | 24 (23–26) | 24 (23–26) | 26 (25–28) | 25 (22–25) | 24 (24–27) |
| VO2 peak (mL/kg/min) | 37 (36–41) | 42 (36–45) | 38 (30–42)§ | 33 (30–38)§ | 29 (26–31)§ |
| Women | 11 F | 11 F | 11 F | 11 F | 11 F |
| Age (years) | 25 (24–27) | 33 (31–37) | 44 (43–46) | 56 (52–59) | 65 (62–66) |
| Height (cm) | 164 (159–167) | 163 (159–165) | 160 (158–165) | 159 (151–165) | 155 (148–162) |
| Weight (kg) | 59.5 (51.9–63.5) | 60.0 (53.1–62.7) | 64.1 (62.0–71.5) | 58.5 (57.1–61.1) | 66.3 (56.5–70.1) |
| BMI (kg/m2) | 22 (20–23) | 22 (20–25) | 25 (24–26)* | 24 (23–25) | 27 (25–27)*§ |
| VO2 peak (mL/kg/min) | 28 (25–31) | 27 (25–36) | 27 (22–29) | 22 (20–25)*§ | 19 (18–21)*§# |
| Experimental Condition
| REST
| STAND
| |||
|---|---|---|---|---|---|
| Index | r | significance | r | significance | |
| All (55 M/55 F) | μHP | 0.027 | No | 0.258 | Yes |
| σ2HP | −0.378 | Yes | −0.433 | Yes | |
| μSAP | 0.287 | Yes | 0.324 | Yes | |
| σ2SAP | 0.339 | Yes | −0.003 | No | |
| Men (55 M) | μHP | −0.037 | No | 0.015 | No |
| σ2HP | −0.415 | Yes | −0.546 | Yes | |
| μSAP | 0.064 | No | 0.153 | No | |
| σ2SAP | 0.220 | No | −0.137 | No | |
| Women (55 F) | μHP | 0.106 | No | 0.579 | Yes |
| σ2HP | −0.348 | Yes | −0.326 | Yes | |
| μSAP | 0.477 | Yes | 0.480 | Yes | |
| σ2SAP | 0.424 | Yes | 0.148 | No | |
| Experimental Condition
| REST
| STAND
| |||
|---|---|---|---|---|---|
| Index | r | significance | r | significance | |
| All (55 M/55 F) | μHP | 0.422 | Yes | 0.394 | Yes |
| σ2HP | 0.343 | Yes | –0.046 | No | |
| μSAP | –0.161 | No | 0.070 | No | |
| σ2SAP | –0.493 | Yes | –0.333 | Yes | |
| Men (55 M) | μHP | 0.585 | Yes | 0.546 | Yes |
| σ2HP | 0.432 | Yes | 0.090 | No | |
| μSAP | –0.143 | No | –0.034 | No | |
| σ2SAP | –0.503 | Yes | –0.550 | Yes | |
| Women (55 F) | μHP | 0.388 | Yes | 0.401 | Yes |
| σ2HP | 0.294 | Yes | –0.127 | No | |
| μSAP | –0.178 | No | 0.109 | No | |
| σ2SAP | –0.5 | Yes | –0.205 | No | |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catai, A.M.; Takahashi, A.C.M.; Perseguini, N.M.; Milan, J.C.; Minatel, V.; Rehder-Santos, P.; Marchi, A.; Bari, V.; Porta, A. Effect of the Postural Challenge on the Dependence of the Cardiovascular Control Complexity on Age. Entropy 2014, 16, 6686-6704. https://doi.org/10.3390/e16126686
Catai AM, Takahashi ACM, Perseguini NM, Milan JC, Minatel V, Rehder-Santos P, Marchi A, Bari V, Porta A. Effect of the Postural Challenge on the Dependence of the Cardiovascular Control Complexity on Age. Entropy. 2014; 16(12):6686-6704. https://doi.org/10.3390/e16126686
Chicago/Turabian StyleCatai, Aparecida M., Anielle C.M. Takahashi, Natália M. Perseguini, Juliana C. Milan, Vinicius Minatel, Patrícia Rehder-Santos, Andrea Marchi, Vlasta Bari, and Alberto Porta. 2014. "Effect of the Postural Challenge on the Dependence of the Cardiovascular Control Complexity on Age" Entropy 16, no. 12: 6686-6704. https://doi.org/10.3390/e16126686
APA StyleCatai, A. M., Takahashi, A. C. M., Perseguini, N. M., Milan, J. C., Minatel, V., Rehder-Santos, P., Marchi, A., Bari, V., & Porta, A. (2014). Effect of the Postural Challenge on the Dependence of the Cardiovascular Control Complexity on Age. Entropy, 16(12), 6686-6704. https://doi.org/10.3390/e16126686