Synthesis
5,10-Dioxo-5H,10H-diimidazo{1,5-a:1´,5´-d}pyrazine-1,6-dicarbonyl Glycyl Esters, 3{1-2}. To a dry round-bottom flask under argon was added dry CH2Cl2 (25 mL). To this stirred solvent at -78 °C were added, in order, the pyrazine diacid chloride 1 (1.00 g, 3.19 mmol), the glycine ester hydrochloride salt 2{1-2} (6.38 mmol) and N,N-diethylaniline (1.91 g, 2.05 mL, 12.8 mmol). The resulting solution was held at -78 °C for 30 minutes before stirring 1 hour at room temperature. The solid precipitate was collected by vacuum filtration and washed with EtOAc to yield 3{1-2}. The final products were characterized by melting point as well as 1H- and 13C-NMR spectroscopy.
3{1}: The product was a white solid obtained in 86% yield: mp > 250 °C; 1H-NMR (DMSO-d6) δ 8.97-8.94 (m, 4 H), 3.97 (d, J = 6.4 Hz, 4 H), 1.43 (s, 18 H); 13C-NMR (DMSO-d6) δ 168.4, 159.2,149.0, 143.9, 138.3, 120.6, 80.9, 41.7, 27.7.
3{2}: The product was a white solid obtained in 91% yield: mp 222-223 °C (dec); 1H-NMR (DMSO-d6) δ 9.07 (t, J = 6.0 Hz, 2 H), 8.97 (d, J = 6.0 Hz, 2 H), 7.39-7.31 (m, 10 H), 5.18 (s, 4H), 4.15 (d, J = 6.0 Hz, 4 H); 13C-NMR (DMSO-d6) δ 169.3, 159.4, 148.9, 143.7, 138.3, 135.8, 128.4, 128.0, 127.9, 120.7, 66.0, 41.1.
5,10-dioxo-5H,10H-diimidazo{1,5-a:1´,5´-d}pyrazine-1,6-dicarbonyl Amino Acid Esters, 3{3-9}. To a dry round-bottom flask under argon was added dry CH2Cl2 (25 mL). To this stirred solvent at -78 °C were added, in order, the pyrazine diacid chloride 1 (1.00 g, 3.19 mmol), the amino acid ester hydrochloride or tosylate salt 2{3-9} (6.38 mmol) and N,N-diethylaniline (1.91 g, 2.05 mL, 12.8 mmol). The resulting solution was held at -78 °C for 30 minutes before stirring 1 hour at room temperature. The reaction was washed against water (3×) and the organic fraction was dried over MgSO4, filtered and concentrated. The residue was suspended in boiling EtOAc, stirred for 15 minutes and then cooled at 0 °C. The solid product 3{3-9} was collected by vacuum filtration and washed with EtOAc. The final product was characterized by melting point as well as 1H- and 13C-NMR spectroscopy.
3{3}: The product was a light yellow solid obtained in 87% yield: mp 225 °C (dec); 1H-NMR (DMSO-d6) δ 8.68 (d, J = 7.6 Hz, 2 H), 8.64 (s, 2 H), 4.71 (quintet, J = 7.2 Hz, 2 H), 1.51 (d, J = 6.8 Hz, 6 H), 1.48 (s, 18 H); 13C-NMR (DMSO-d6) δ 171.6, 157.2, 149.2, 146.9, 138.3, 118.9, 82.3, 49.2, 27.9, 18.4.
3{4}: The product was a yellow solid obtained in 93% yield: mp 141-143 °C; 1H-NMR (DMSO-d6) δ 8.68 (d, J = 7.2 Hz, 2 H), 8.62 (s, 2 H), 7.35-7.31 (m, 10 H), 5.21 (dd, J = 12 Hz, J = 12 Hz, 4 H), 4.87 (quintet, J = 7.2 Hz, 2 H), 1.56 (d, J = 7.2 Hz, 6 H); 13C-NMR (DMSO-d6) δ 172.2, 157.6, 148.9, 146.7, 138.4, 135.2, 128.6, 128.5, 128.2, 118.9, 67.4, 48.8, 18.3.
3{5}: The product was a white solid obtained in 83% yield: mp 222-223 °C (dec); 1H-NMR (DMSO-d6) δ 8.63 (s, 2 H), 8.51 (d, J = 8 Hz, 2 H), 4.79-7.74 (m, 2 H), 1.77-1.65 (m, 3.22 6 H), 1.47 (s, 18 H), 0.97 (d, J = 6.4 Hz, 12 H); 13C-NMR (DMSO-d6) δ 171.6, 157.4, 149.0, 147.1, 138.4, 118.8, 82.3, 52.0, 41.9, 28.0, 25.1, 22.8, 22.2.
3{6}: The product was a white solid obtained in 87% yield: mp 176-178 °C (Lit. 140-141 °C, Ref. [
16]);
1H-NMR (DMSO-
d6) δ 8.62 (s, 2 H), 8.52 (d,
J = 8.4 Hz, 2 H), 7.35-7.29 (m, 10 H), 5.18 (s, 4 H), 4.94-4.88 (m, 2 H), 1.82-1.69 (m, 6 H), 0.95 (d,
J = 2.4 Hz, 6 H); 0.94 (d,
J = 2.0 Hz, 6 H);
13C- NMR (DMSO-
d6) δ 172.1, 157.6, 149.0, 146.6, 138.4, 135.3, 128.6, 128.4, 128.2, 119.0, 67.2, 51.5, 41.4, 25.0, 22.8, 22.2.
3{7}: The product was a white solid obtained in 87% yield: mp 201-203 °C (dec) (Lit. 179-180 °C (dec) [
10]);
1H-NMR (DMSO-
d6) δ 8.59 (d,
J = 4 Hz, 2 H), 8.57 (s, 2 H), 7.27-7.19 (m, 10 H), 5.00 (dd,
J = 6.4 Hz,
J = 6.4 Hz, 2 H), 3.22 (d,
J = 6.4 Hz, 4 H), 1.41 (s, 18 H);
13C-NMR (DMSO-
d6) δ 170.0, 157.3, 148.9, 146.7, 138.3, 136.0, 129.4, 128.3, 126.9, 118.8, 82.6, 54.3, 38.1, 27.9.
3{8}: The product was a white solid obtained in 86% yield: mp 175-177 °C; 1H-NMR (DMSO-d6) δ 8.56 (d, J = 8 Hz, 2 H), 8.53 (s, 2 H), 7.34-7.04 (m, 20 H), 5.15-5.10 (m, 6 H), 3.27 (dd, J = 6.0 Hz, J = 14, 2 H), 3.20 (dd, J = 6.0 Hz, J = 13.6, 2 H); 13C-NMR (DMSO-d6) δ 170.8, 157.4, 148.7, 146.4, 138.3, 135.5, 135.0, 129.3, 128.5, 127.1, 118.9, 67.4, 53.9, 37.8.
3{9}: The product was a white solid obtained in 76% yield: mp 150-152 °C; 1H-NMR (DMSO-d6) δ 8.71-8.68 (m, 4 H), 4.78-4.73 (m, 2 H), 4.49 (broad, 2 H), 3.08 (bs, 4 H), 2.01-1.94 (m, 2 H), 1.85-1.78 (m, 2 H), 1.53-1.32 (m, 44 H); 13C-NMR (DMSO-d6) δ 170.8, 157.5, 155.9, 149.2, 146.9, 138.6, 118.8, 82.6, 79.2, 53.2, 40.4, 32.1, 29.7, 28.4, 28.02, 28.0, 22.3.
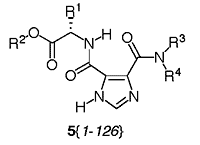
Dissymmetric Imidazole-4,5-dicarboxamides 5{2-6}, 5{8-13}, 5{16-20}, 5{22-27}, 5{30-34}, 5{36-41}, 5{44-48}, 5{50-55}, 5{58-62}, 5{64-69}, 5{72-76}, 5{78-83}, 5{86-90}, 5{92-97}, 5{100-104}, 5{106-111}, 5{114-118}, 5{120-125}, Disubstituted with Amino Acid Esters 2{1-9} and Alkanamines 4 {2-6}, 4{8-13}. Screw capped culture tubes were dried overnight in an oven. Each of the nine amino acid ester substituted pyrazine 3{1-9} (0.100 mmol) was transferred to 11 culture tubes followed by dry CH2Cl2 (3 mL). Solutions of the eleven different amines 4{2-6} and 4{8-13} at 4 M were prepared in CH2Cl2 and 50 mL (0.200 mmol) of each amine was transferred robotically to the corresponding tubes for a total of 99 tubes (9×11). The tubes were purged with argon and capped. The tubes were placed on an orbital shaker for 2 days at room temperature and the solutions gently mixed by the orbital action. The progress of the reactions was followed with TLC by using a mixture of EtOAc/hexane (1:1) as the eluant. After 2 days the solvent was evaporated and the residues were purified by column chromatography on silica gel with EtOAc/hexane (1:1) initially and then just EtOAc as the eluant. The desired fractions were combined and concentrated to give the final products. The purity and identity of the final products was tested by LC-MS and 1H-NMR.
Dissymmetric Imidazole-4,5-dicarboxamides 5{1}, 5{15}, 5{29}, 5{43}, 5{57}, 5{71}, 5{85}, 5{99}, 5{113} Disubstituted with Amino Acid Esters 2{1-9} and Methylamine Hydrochloride 4{1}. Screw capped culture tubes were dried overnight in an oven. Each tube was filled with one amino acid ester substituted pyrazine 3{1-9} (0.100 mmol). Methylamine hydrochloride 4{1} (27.0 mg, 0.400 mmol) was then weighed and transferred into each tube, followed by dry CH2Cl2 (3 mL) for a total of 9 tubes (9×1). A 4.0 M solution of diisopropylethylamine (DIEA) in CH2Cl2 was prepared and 100 mL (0.400 mmol) of this solution was added to each tube. The tubes were purged with argon and sealed. The tubes were placed on an orbital shaker for 2 days at room temperature and the solutions gently mixed by the orbital action. The progress of the reactions was followed with TLC by using a mixture of EtOAc/hexane (1:1) as the eluant. After 2 days the solvent was evaporated and the residues were purified by column chromatography on silica gel with EtOAc/hexane (1:1) initially and then just EtOAc as the eluant. The desired fractions were combined and concentrated to give the final products. The purity and identity of the final products was tested by LC-MS and 1H-NMR.
Dissymmetric Imidazole-4,5-dicarboxamides 5{7}, 5{21}, 5{35}, 5{49}, 5{63}, 5{77}, 5{91}, 5{105}, 5{119} Disubstituted with Amino Acid Esters 2{1-9} and 1-(N-Boc-aminomethyl)-4-(aminomethyl)benzene 4{7}. Screw capped culture tubes were dried overnight in an oven. Each tube was filled with one amino acid ester substituted pyrazine 3{1-9} (0.100 mmol). 1-(N-Boc-aminomethyl)-4-(aminomethyl)benzene 4{7} (47.3 mg, 0.200 mmol) was then weighed and transferred into each tube, followed by dry CH2Cl2 (3 mL) for a total of 9 tubes (9×1). The tubes were purged with argon and sealed. The tubes were placed on an orbital shaker for 2 days at room temperature and the solutions gently mixed by the orbital action. The progress of the reactions was followed with TLC by using a mixture of EtOAc/hexane (1:1) as the eluant. After 2 days the solvent was evaporated and the residues were purified by column chromatography on silica gel with EtOAc/hexane (1:1) initially and then just EtOAc as the eluant. The desired fractions were combined and concentrated to give the final products. The purity and identity of the final products was tested by LC-MS and 1H-NMR.
Dissymmetric Imidazole-4,5-dicarboxamides 5{14}, 5{28}, 5{42}, 5{56}, 5{70}, 5{84}, 5{98}, 5{112}, 5{126} Disubstituted with Amino Acid Esters 2{1-9} and 1-Phenylpiperazine Hydrochloride 4{14}. Screw capped culture tubes were dried overnight in an oven. Each tube was filled with one amino acid ester substituted pyrazine 3{1-9} (0.100 mmol). 1-Phenylpiperazine hydrochloride 4{14} (39.7 mg, 0.200 mmol) was then weighed and transferred into each tube, followed by dry CH2Cl2 (3 mL) for a total of 9 tubes (9×1). A 4.0 M solution of diisopropylethylamine (DIEA) in CH2Cl2 was prepared and 50 mL (0.200 mmol) of this solution was added to each tube. The tubes were purged with argon and sealed. The tubes were placed on an orbital shaker for 2 days at room temperature and the solutions gently mixed by the orbital action. The progress of the reactions was followed with TLC by using a mixture of EtOAc/hexane (1:1) as the eluant. After 2 days the solvent was evaporated and the residues were purified by column chromatography on silica gel with EtOAc/hexane (1:1) initially and then just EtOAc as the eluant. The desired fractions were combined and concentrated to give the final products. The purity and identity of the final products was tested by LC-MS and 1H-NMR.
Table 7.
Final Product Numbers and Nomenclature.
Table 7.
Final Product Numbers and Nomenclature.
| cmpd | Name |
|---|
| 5{1} | 4-[(methylamino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{2} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{3} | 4-[(benzylamino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{4} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{5} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{6} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{7} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{8} | 4-[(dibenzylamino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{9} | 4-[(N-methylbenzylamino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{10} | 4-[(N,N-diethylamino)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{11} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{12} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{13} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{14} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxyglycyl)carbonyl]-1H-imidazole |
| 5{15} | 4-[(methylamino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1
H-imidazole |
| 5{16} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1
H-imidazole |
| 5{17} | 4-[(benzylamino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1
H-imidazole |
| 5{18} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 5{19} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 5{20} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1
H-imidazole |
| 5{21} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 5{22} | 4-[(dibenzylamino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1
H-imidazole |
| 5{23} | 4-[(N-methylbenzylamino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 5{24} | 4-[(N,N-diethylamino)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 5{25} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 5{26} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1
H-imidazole |
| 5{27} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(benzyloxyglycyl)carbonyl]-1
H-imidazole |
| 5{28} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(benzyloxyglycyl)carbonyl]-1H-imidazole |
| 5{29} | 4-[(methylamino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{30} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{31} | 4-[(benzylamino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{32} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{33} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{34} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{35} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{36} | 4-[(dibenzylamino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{37} | 4-[(N-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{38} | 4-[(N,N-diethylamino)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{39} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{40} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{41} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{42} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{43} | 4-[(methylamino)carbonyl]-5-[(benzyloxy-
S-alanyl)carbonyl]-1H-imidazole |
| 5{44} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(benzyloxy-
S-alanyl)carbonyl]-1H-imidazole |
| 5{45} | 4-[(benzylamino)carbonyl]-5-[(benzyloxy-
S-alanyl)carbonyl]-1H-imidazole |
| 5{46} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{47} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{48} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(benzyloxy-
S-alanyl)carbonyl]-1H-imidazole |
| 5{49} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{50} | 4-[(dibenzylamino)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1
H-imidazole |
| 5{51} | 4-[(N-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{52} | 4-[(N,N-diethylamino)carbonyl]-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{53} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{54} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(benzyloxy-
S-alanyl)carbonyl]-1H-imidazole |
| 5{55} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(benzyloxy-
S-alanyl)carbonyl]-1H-imidazole |
| 5{56} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(benzyloxy-S-alanyl)carbonyl]-1H-imidazole |
| 5{57} | 4-[(methylamino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{58} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{59} | 4-[(benzylamino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{60} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{61} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{62} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{63} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{64} | 4-[(dibenzylamino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{65} | 4-[(N-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{66} | 4-[(N,N-diethylamino)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{67} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{68} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{69} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{70} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{71} | 4-[(methylamino)carbonyl]-5-[(benzyloxy-
S-leucyl)carbonyl]-1H-imidazole |
| 5{72} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(benzyloxy-
S-leucyl)carbonyl]-1H-imidazole |
| 5{73} | 4-[(benzylamino)carbonyl]-5-[(benzyloxy-
S-leucyl)carbonyl]-1H-imidazole |
| 5{74} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{75} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{76} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(benzyloxy-
S-leucyl)carbonyl]-1H-imidazole |
| 5{77} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{78} | 4-[(dibenzylamino)carbonyl]-5-[(benzyloxy-S-leucyl)carbonyl]-1
H-imidazole |
| 5{79} | 4-[(N-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{80} | 4-[(N,N-diethylamino)carbonyl]-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{81} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{82} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(benzyloxy-
S-leucyl)carbonyl]-1H-imidazole |
| 5{83} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(benzyloxy-
S-leucyl)carbonyl]-1H-imidazole |
| 5{84} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(benzyloxy-S-leucyl)carbonyl]-1H-imidazole |
| 5{85} | 4-[(methylamino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{86} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{87} | 4-[(benzylamino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{88} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{89} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{90} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{91} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{92} | 4-[(dibenzylamino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{93} | 4-[(N-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{94} | 4-[(N,N-diethylamino)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{95} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{96} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{97} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{98} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{99} | 4-[(methylamino)carbonyl]-5-[(benzyloxy-
S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{100} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(benzyloxy-
S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{101} | 4-[(benzylamino)carbonyl]-5-[(benzyloxy-
S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{102} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{103} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{104} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(benzyloxy-
S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{105} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{106} | 4-[(dibenzylamino)carbonyl]-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1
H-imidazole |
| 5{107} | 4-[(N-methylbenzylamino)carbonyl]-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{108} | 4-[(N,N-diethylamino)carbonyl]-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{109} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{110} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(benzyloxy-
S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{111} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(benzyloxy-
S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{112} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(benzyloxy-S-phenylalanyl)carbonyl]-1H-imidazole |
| 5{113} | 4-[(methylamino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{114} | 4-[5-({[(tert-butoxy)carbonyl]amino}pentylamino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{115} | 4-[(benzylamino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{116} | 4-[(R-α-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{117} | 4-[(S-α-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{118} | 4-[4-({[(tert-butoxy)carbonyl]piperidinyl}amino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{119} | 4-{[(4-{[(tert-butoxy)carbonyl]aminomethyl}phenyl)methylamino]carbonyl}-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{120} | 4-[(dibenzylamino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{121} | 4-[(N-methylbenzylamino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{122} | 4-[(N,N-diethylamino)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{123} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{124} | 4-[(1,2,3,4-tetrahydroisoquinolinyl)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{125} | 4-[(4-methylpiperidinyl)carbonyl]-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |
| 5{126} | 4-({4-[(tert-butoxy)carbonyl]piperazinyl}carbonyl)-5-[(tert-butoxy-S-[Ne -(tert-butoxy)carbonyl]lysyl)carbonyl]-1H-imidazole |