Phospholipids: Key Players in Apoptosis and Immune Regulation
Abstract
:1. Introduction
2. Phospholipids in Biological Membranes

3. Major Membrane Changes during Early Apoptosis
3.1. PS translocation in apoptosis
3.2. Cardiolipin translocation in apoptosis

4. How Does Apoptotic Cell Clearance Occur?
- (1) Attraction: finding of apoptotic cells by phagocytes through the recognition of apoptotic cell-derived chemo-attractants (“find me” signals).
- (2) Recognition and engulfment: identification of the abnormal membrane changes like PS exposure with higher lateral mobility, modifications in the glycosilation pattern of the glycocalix, and/or binding of specific bridging molecules (“eat me” signals). A positive recognition leads to phagocytosis, inspection (checking for pathogens), and degradation of the engulfed material.
- (3) Immune down regulation: production of anti-inflammatory cytokines (“tolerate me” signals like IL-10 and TGF-ß).
4.1. Attraction of the phagocyte: Phospholipids as “find me” signals
4.2. Recognition of apoptotic cells: PS as “eat me” signal

4.3. Immune down regulation after uptake of apoptotic cells
4.4. Consequences of a failure in the clearance of apoptotic cells
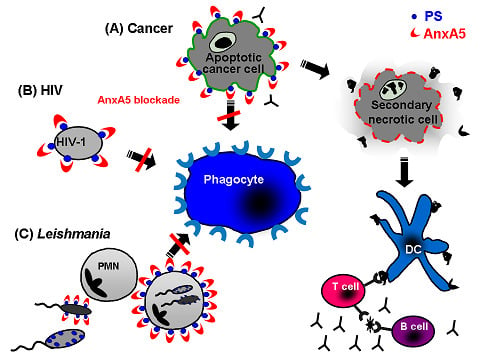
5. Annexin A5: A Natural Ligand of PS
5.1. Annexin A5 in cancer therapy
5.2. Annexin A5 in infections

6. Final Perspectives

Acknowledgements
- Sample Availability: Samples of the compound annexinA5 are available from the authors.
References
- Lenoir, G.; Williamson, P.; Holthuis, J.C. On the origin of lipid asymmetry: the flip side of ion transport. Curr. Opin. Chem. Biol. 2007, 11, 654–661. [Google Scholar] [CrossRef]
- Zachowski, A. Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochem. J. 1993, 294, 1–14. [Google Scholar]
- Vance, J.E. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J. Lipid. Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; van Rijn, J.L.; Hemker, H.C.; Zwaal, R.F. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur. J. Biochem. 1982, 122, 429–436. [Google Scholar] [CrossRef]
- Schroit, A.J.; Zwaal, R.F. Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim. Biophys. Acta 1991, 1071, 313–329. [Google Scholar] [CrossRef]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar]
- Appelt, U.; Sheriff, A.; Gaipl, U.S.; Kalden, J.R.; Voll, R.E.; Herrmann, M. Viable, apoptotic and necrotic monocytes expose phosphatidylserine: cooperative binding of the ligand Annexin V to dying but not viable cells and implications for PS-dependent clearance. Cell Death Differ. 2005, 12, 194–196. [Google Scholar] [CrossRef]
- Callahan, M. K.; Williamson, P.; Schlegel, R. A. Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 2000, 7, 645–653. [Google Scholar] [CrossRef]
- Yoshida, H.; Kawane, K.; Koike, M.; Mori, Y.; Uchiyama, Y.; Nagata, S. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature 2005, 437, 754–758. [Google Scholar] [CrossRef]
- Dillon, S.R.; Mancini, M.; Rosen, A.; Schlissel, M.S. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J. Immunol. 2000, 164, 1322–1332. [Google Scholar]
- Cocco, L.; Martelli, A.M.; Billi, A.M.; Cataldi, A.; Miscia, S.; Mottola, M.R.; Manzoli, L. Phospholipids as components of the nuclear matrix: their possible biological significance. Basic Appl. Histochem. 1987, 31, 413–419. [Google Scholar]
- Furnrohr, B.G.; Groer, G.J.; Sehnert, B.; Herrmann, M.; Voll, R.E. Interaction of histones with phospholipids-implications for the exposure of histones on apoptotic cells. Autoimmunity 2007, 40, 322–326. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Cancer cell immune escape and tumor progression by exploitation of anti-inflammatory and pro-inflammatory responses. Cancer Biol. Ther. 2005, 4, 924–933. [Google Scholar] [CrossRef]
- Drucker, L.; Ciobotaro, P.; Kimchi, O.; Tohami, T.; Yarkoni, S.; Radnay, J.; Shapira, H.; Lishner, M. Initial exposed phosphatidylserine levels correlate with cellular response to cytotoxic drugs. Eur. J. Haematol. 2003, 70, 98–105. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K.; Arihiro, K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006, 66, 5527–5536. [Google Scholar] [CrossRef]
- Ran, S.; Thorpe, P. E. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1479–1484. [Google Scholar] [CrossRef]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Martin, S.J.; Reutelingsperger, C.P.; McGahon, A.J.; Rader, J.A.; van Schie, R.C.; LaFace, D. M.; Green, D. R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995, 182, 1545–1556. [Google Scholar] [CrossRef]
- Verhoven, B.; Schlegel, R.A.; Williamson, P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 1995, 182, 1597–1601. [Google Scholar] [CrossRef]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar]
- van den Eijnde, S.M.; Boshart, L.; Baehrecke, E.H.; De Zeeuw, C.I.; Reutelingsperger, C.P.; Vermeij-Keers, C. Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis 1998, 3, 9–16. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Strayer, L. Biochemistry, 5th ed; Spektrum, Akad. Verl.: Berlin, Germany, 2003. [Google Scholar]
- Wang, X.; Devaiah, S.P.; Zhang, W.; Welti, R. Signaling functions of phosphatidic acid. Prog. Lipid Res. 2006, 45, 250–278. [Google Scholar] [CrossRef]
- Stace, C.L.; Ktistakis, N.T. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim. Biophys. Acta 2006, 1761, 913–926. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Yamaji-Hasegawa, A.; Tsujimoto, M. Asymmetric distribution of phospholipids in biomembranes. Biol. Pharm. Bull. 2006, 29, 1547–1553. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.Y.; Shi, Y. Identification and functional characterization of hCLS1, a human cardiolipin synthase localized in mitochondria. Biochem. J. 2006, 398, 169–176. [Google Scholar] [CrossRef]
- Schlame, M.; Rua, D.; Greenberg, M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000, 39, 257–288. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Tang, X.; Halleck, M.S.; Schlegel, R.A.; Williamson, P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 1996, 272, 1495–1497. [Google Scholar]
- Williamson, P.; Schlegel, R.A. Transbilayer phospholipid movement and the clearance of apoptotic cells. Biochim. Biophys. Acta 2002, 1585, 53–63. [Google Scholar] [CrossRef]
- Folmer, D.E.; Elferink, R.P.; Paulusma, C.C. P4 ATPases - lipid flippases and their role in disease. Biochim. Biophys. Acta 2009, 1791, 628–635. [Google Scholar] [CrossRef]
- Muthusamy, B.P.; Natarajan, P.; Zhou, X.; Graham, T.R. Linking phospholipid flippases to vesicle-mediated protein transport. Biochim. Biophys. Acta 2009, 1791, 612–619. [Google Scholar] [CrossRef]
- Riekhof, W.R.; Voelker, D.R. The yeast plasma membrane P4-ATPases are major transporters for lysophospholipids. Biochim. Biophys. Acta 2009, 1791, 620–627. [Google Scholar] [CrossRef]
- Sahu, S.K.; Gummadi, S.N.; Manoj, N.; Aradhyam, G.K. Phospholipid scramblases: an overview. Arch. Biochem. Biophys. 2007, 462, 103–114. [Google Scholar] [CrossRef]
- Higgins, C.F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef]
- Stefkova, J.; Poledne, R.; Hubacek, J.A. ATP-binding cassette (ABC) transporters in human metabolism and diseases. Physiol. Res. 2004, 53, 235–243. [Google Scholar]
- Klappe, K.; Hummel, I.; Hoekstra, D.; Kok, J.W. Lipid dependence of ABC transporter localization and function. Chem. Phys. Lipids 2009, 161, 57–64. [Google Scholar] [CrossRef]
- Hamon, Y.; Broccardo, C.; Chambenoit, O.; Luciani, M. F.; Toti, F.; Chaslin, S.; Freyssinet, J. M.; Devaux, P.F.; McNeish, J.; Marguet, D.; Chimini, G. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2000, 2, 399–406. [Google Scholar] [CrossRef]
- Palade, G.E. The fine structure of mitochondria. Anat. Rec. 1952, 114, 427–451. [Google Scholar] [CrossRef]
- Palade, G.E. An electron microscope study of the mitochondrial structure. J. Histochem. Cytochem. 1953, 1, 188–211. [Google Scholar] [CrossRef]
- Sjostrand, F.S. Electron microscopy of mitochondria and cytoplasmic double membranes. Nature 1953, 171, 30–32. [Google Scholar] [CrossRef]
- Sjostrand, F.S. Morphology of ordered biological structures. Radiat. Res. 1960, Suppl. 2, 349–386. [Google Scholar]
- Krebs, J.J.; Hauser, H.; Carafoli, E. Asymmetric distribution of phospholipids in the inner membrane of beef heart mitochondria. J. Biol. Chem. 1979, 254, 5308–5316. [Google Scholar]
- Daum, G. Lipids of mitochondria. Biochim. Biophys. Acta 1985, 822, 1–42. [Google Scholar] [CrossRef]
- Hovius, R.; Lambrechts, H.; Nicolay, K.; de Kruijff, B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim. Biophys. Acta 1021, 1990, 217–226. [Google Scholar]
- Simbeni, R.; Pon, L.; Zinser, E.; Paltauf, F.; Daum, G. Mitochondrial membrane contact sites of yeast. Characterization of lipid components and possible involvement in intramitochondrial translocation of phospholipids. J. Biol. Chem. 1991, 266, 10047–10049. [Google Scholar]
- Zinser, E.; Sperka-Gottlieb, C.D.; Fasch, E.V.; Kohlwein, S.D.; Paltauf, F.; Daum, G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 1991, 173, 2026–2034. [Google Scholar]
- Gonzalvez, F.; Gottlieb, E. Cardiolipin: setting the beat of apoptosis. Apoptosis 2007, 12, 877–885. [Google Scholar] [CrossRef]
- de Kroon, A.I.; Dolis, D.; Mayer, A.; Lill, R.; de Kruijff, B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim. Biophys. Acta 1997, 1325, 108–116. [Google Scholar] [CrossRef]
- Ardail, D.; Lerme, F.; Louisot, P. Further characterization of mitochondrial contact sites: effect of short-chain alcohols on membrane fluidity and activity. Biochem. Biophys. Res. Commun. 1990, 173, 878–885. [Google Scholar] [CrossRef]
- Smith, D.J.; Ng, H.; Kluck, R.M.; Nagley, P. The mitochondrial gateway to cell death. IUBMB Life 2008, 60, 383–389. [Google Scholar] [CrossRef]
- Garcia Fernandez, M.; Troiano, L.; Moretti, L.; Nasi, M.; Pinti, M.; Salvioli, S.; Dobrucki, J.; Cossarizza, A. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ. 2002, 13, 449–455. [Google Scholar]
- Schug, Z.T.; Gottlieb, E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim. Biophys. Acta 2009, 1788, 2022–2031. [Google Scholar] [CrossRef]
- Degli Esposti, M. Sequence and functional similarities between pro-apoptotic Bid and plant lipid transfer proteins. Biochim. Biophys. Acta 2002, 1553, 331–340. [Google Scholar] [CrossRef]
- Esposti, M.D.; Erler, J. T.; Hickman, J.A.; Dive, C. Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity. Mol. Cell Biol. 2001, 21, 7268–7276. [Google Scholar] [CrossRef]
- Sorice, M.; Circella, A.; Cristea, I.M.; Garofalo, T.; Di Renzo, L.; Alessandri, C.; Valesini, G.; Esposti, M.D. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004, 11, 1133–1145. [Google Scholar] [CrossRef]
- Tyurin, V.A.; Tyurina, Y.Y.; Osipov, A.N.; Belikova, N.A.; Basova, L.V.; Kapralov, A.A.; Bayir, H.; Kagan, V.E. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Differ. 2007, 14, 872–875. [Google Scholar] [CrossRef]
- Epand, R.F.; Martinou, J.C.; Montessuit, S.; Epand, R.M. Transbilayer lipid diffusion promoted by Bax: implications for apoptosis. Biochemistry 2003, 42, 14576–14582. [Google Scholar] [CrossRef]
- Schlattner, U.; Tokarska-Schlattner, M.; Ramirez, S.; Bruckner, A.; Kay, L.; Polge, C.; Epand, R.F.; Lee, R.M.; Lacombe, M.L.; Epand, R.M. Mitochondrial kinases and their molecular interaction with cardiolipin. Biochim. Biophys. Acta 2009, 1788, 2032–2047. [Google Scholar] [CrossRef]
- Epand, R.F.; Schlattner, U.; Wallimann, T.; Lacombe, M.L.; Epand, R.M. Novel lipid transfer property of two mitochondrial proteins that bridge the inner and outer membranes. Biophys. J. 2007, 92, 126–137. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Dai, Q.; Lee, R. M. Phospholipid scramblase 3 is the mitochondrial target of protein kinase C delta-induced apoptosis. Cancer Res. 2003, 63, 1153–1156. [Google Scholar]
- Liu, J.; Dai, Q.; Chen, J.; Durrant, D.; Freeman, A.; Liu, T.; Grossman, D.; Lee, R.M. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol. Cancer Res. 2003, 1, 892–902. [Google Scholar]
- Lutter, M.; Fang, M.; Luo, X.; Nishijima, M.; Xie, X.; Wang, X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000, 2, 754–761. [Google Scholar] [CrossRef]
- Wei, M. C.; Lindsten, T.; Mootha, V. K.; Weiler, S.; Gross, A.; Ashiya, M.; Thompson, C. B.; Korsmeyer, S. J. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000, 14, 2060–2071. [Google Scholar]
- Kim, T.H.; Zhao, Y.; Ding, W.X.; Shin, J.N.; He, X.; Seo, Y.W.; Chen, J.; Rabinowich, H.; Amoscato, A.A.; Yin, X.M. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Mol. Biol. Cell 2004, 15, 3061–3072. [Google Scholar] [CrossRef]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; Vlasova, II; Zhao, Q.; Zou, M.; Di, P.; Svistunenko, D.A.; Kurnikov, I.V.; Borisenko, G.G. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005, 1, 223–232. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Schug, Z.T.; Houtkooper, R.H.; MacKenzie, E.D.; Brooks, D.G.; Wanders, R.J.; Petit, P.X.; Vaz, F.M.; Gottlieb, E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J. Cell Biol. 2008, 183, 681–696. [Google Scholar] [CrossRef]
- Sorice, M.; Manganelli, V.; Matarrese, P.; Tinari, A.; Misasi, R.; Malorni, W.; Garofalo, T. Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett. 2009, 583, 2447–2450. [Google Scholar] [CrossRef]
- Lauber, K.; Bohn, E.; Krober, S.M.; Xiao, Y.J.; Blumenthal, S.G.; Lindemann, R. K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S.; Xu, Y.; Autenrieth, I.B.; Schulze-Osthoff, K.; Belka, C.; Stuhler, G.; Wesselborg, S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef]
- Peter, C.; Waibel, M.; Radu, C.G.; Yang, L.V.; Witte, O.N.; Schulze-Osthoff, K.; Wesselborg, S.; Lauber, K. Migration to apoptotic "find-me" signals is mediated via the phagocyte receptor G2A. J. Biol. Chem. 2008, 283, 5296–5305. [Google Scholar]
- Mueller, R.B.; Sheriff, A.; Gaipl, U.S.; Wesselborg, S.; Lauber, K. Attraction of phagocytes by apoptotic cells is mediated by lysophosphatidylcholine. Autoimmunity 2007, 40, 342–344. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Gude, D.R.; Alvarez, S. E.; Paugh, S.W.; Mitra, P.; Yu, J.; Griffiths, R.; Barbour, S.E.; Milstien, S.; Spiegel, S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a "come-and-get-me" signal. FASEB J. 2008, 22, 2629–2638. [Google Scholar] [CrossRef]
- Schlegel, R.A.; Williamson, P. Phosphatidylserine, a death knell. Cell Death Differ. 2001, 8, 551–563. [Google Scholar] [CrossRef]
- Bottcher, A.; Gaipl, U.S.; Furnrohr, B.G.; Herrmann, M.; Girkontaite, I.; Kalden, J.R.; Voll, R.E. Involvement of phosphatidylserine, alphavbeta3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophas. Arthritis Rheum. 2006, 54, 927–938. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Lorenz, U. Engulfment of apoptotic cells: signals for a good meal. Nat. Rev. Immunol. 2007, 7, 964–974. [Google Scholar] [CrossRef]
- Umeda, M.; Igarashi, K.; Nam, K.S.; Inoue, K. Effective production of monoclonal antibodies against phosphatidylserine: stereo-specific recognition of phosphatidylserine by monoclonal antibody. J. Immunol. 1989, 143, 2273–2279. [Google Scholar]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a factor that links apoptotic cells to phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef]
- Scott, R. S.; McMahon, E. J.; Pop, S. M.; Reap, E. A.; Caricchio, R.; Cohen, P. L.; Earp, H. S.; Matsushima, G. K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001, 411, 207–211. [Google Scholar]
- Balasubramanian, K.; Schroit, A.J. Characterization of phosphatidylserine-dependent beta2-glycoprotein I macrophage interactions. Implications for apoptotic cell clearance by phagocytes. J. Biol. Chem. 1998, 273, 29272–29277. [Google Scholar] [CrossRef]
- Lutz, H.U. Innate immune and non-immune mediators of erythrocyte clearance. Cell. Mol. Biol. (Noisy-le-grand) 2004, 50, 107–116. [Google Scholar]
- Anderson, H.A.; Maylock, C.A.; Williams, J.A.; Paweletz, C.P.; Shu, H.; Shacter, E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat. Immunol. 2003, 4, 87–91. [Google Scholar]
- Arur, S.; Uche, U.E.; Rezaul, K.; Fong, M.; Scranton, V.; Cowan, A.E.; Mohler, W.; Han, D.K. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 2003, 4, 587–598. [Google Scholar] [CrossRef]
- Rigotti, A.; Acton, S.L.; Krieger, M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 1995, 270, 16221–16224. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Rose, D.M.; Pearson, A.; Ezekewitz, R.A.; Henson, P.M. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 2000, 405, 85–90. [Google Scholar]
- Bose, J.; Gruber, A.D.; Helming, L.; Schiebe, S.; Wegener, I.; Hafner, M.; Beales, M.; Kontgen, F.; Lengeling, A. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J. Biol. 2004, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.E.; Cvetanovic, M.; Tibrewal, N.; Patel, V.; Colamonici, O.R.; Li, M.O.; Flavell, R.A.; Levine, J.S.; Birge, R.B.; Ucker, D.S. The presumptive phosphatidylserine receptor is dispensable for innate anti-inflammatory recognition and clearance of apoptotic cells. J. Biol. Chem. 2006, 281, 5718–5725. [Google Scholar]
- Miyanishi, M.; Tada, K.; Koike, M.; Uchiyama, Y.; Kitamura, T.; Nagata, S. Identification of Tim4 as a phosphatidylserine receptor. Nature 2007, 450, 435–439. [Google Scholar] [CrossRef]
- Kobayashi, N.; Karisola, P.; Pena-Cruz, V.; Dorfman, D.M.; Jinushi, M.; Umetsu, S.E.; Butte, M.J.; Nagumo, H.; Chernova, I.; Zhu, B.; Sharpe, A.H.; Ito, S.; Dranoff, G.; Kaplan, G.G.; Casasnovas, J.M.; Umetsu, D.T.; Dekruyff, R.H.; Freeman, G.J. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 2007, 27, 927–940. [Google Scholar] [CrossRef]
- Santiago, C.; Ballesteros, A.; Tami, C.; Martinez-Munoz, L.; Kaplan, G.G.; Casasnovas, J.M. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity 2007, 26, 299–310. [Google Scholar] [CrossRef]
- Adachi, H.; Tsujimoto, M. FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. J. Biol. Chem. 2002, 277, 34264–34270. [Google Scholar] [CrossRef]
- Tamura, Y.; Adachi, H.; Osuga, J.; Ohashi, K.; Yahagi, N.; Sekiya, M.; Okazaki, H.; Tomita, S.; Iizuka, Y.; Shimano, H.; Nagai, R.; Kimura, S.; Tsujimoto, M.; Ishibashi, S. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J. Biol. Chem. 2003, 278, 12613–12617. [Google Scholar]
- Falkowski, M.; Schledzewski, K.; Hansen, B.; Goerdt, S. Expression of stabilin-2, a novel fasciclin-like hyaluronan receptor protein, in murine sinusoidal endothelia, avascular tissues, and at solid/liquid interfaces. Histochem. Cell Biol. 2003, 120, 361–369. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, M.Y.; Kim, H.J.; Lee, S.J.; Kim, S.Y.; Lee, B.H.; Kwon, T.H.; Park, R.W.; Kim, I.S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008, 15, 192–201. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.Y.; Jung, M.Y.; Bae, D.J.; Kim, I.S. Epidermal growth factor-like domain repeat of stabilin-2 recognizes phosphatidylserine during cell corpse clearance. Mol. Cell. Biol. 2008, 28, 5288–5298. [Google Scholar] [CrossRef]
- Park, D.; Tosello-Trampont, A.C.; Elliott, M.R.; Lu, M.; Haney, L.B.; Ma, Z.; Klibanov, A.L.; Mandell, J.W.; Ravichandran, K. S. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 2007, 450, 430–434. [Google Scholar] [CrossRef]
- Gumienny, T.L.; Brugnera, E.; Tosello-Trampont, A.C.; Kinchen, J.M.; Haney, L.B.; Nishiwaki, K.; Walk, S.F.; Nemergut, M.E.; Macara, I.G.; Francis, R.; Schedl, T.; Qin, Y.; Van Aelst, L.; Hengartner, M.O.; Ravichandran, K. S. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 2001, 107, 27–41. [Google Scholar] [CrossRef]
- Brugnera, E.; Haney, L.; Grimsley, C.; Lu, M.; Walk, S.F.; Tosello-Trampont, A.C.; Macara, I. G.; Madhani, H.; Fink, G.R.; Ravichandran, K. S. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 2002, 4, 574–582. [Google Scholar]
- Lu, M.; Ravichandran, K.S. Dock180-ELMO cooperation in Rac activation. Methods Enzymol. 2006, 406, 388–402. [Google Scholar] [CrossRef]
- Bjarnadottir, T.K.; Fredriksson, R.; Hoglund, P.J.; Gloriam, D.E.; Lagerstrom, M.C.; Schioth, H. B. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics 2004, 84, 23–33. [Google Scholar]
- Nishimori, H.; Shiratsuchi, T.; Urano, T.; Kimura, Y.; Kiyono, K.; Tatsumi, K.; Yoshida, S.; Ono, M.; Kuwano, M.; Nakamura, Y.; Tokino, T. A novel brain-specific p53-target gene, BAI1, containing thrombospondin type 1 repeats inhibits experimental angiogenesis. Oncogene 1997, 15, 2145–2150. [Google Scholar]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive effects of apoptotic cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef]
- Lucas, M.; Stuart, L.M.; Zhang, A.; Hodivala-Dilke, K.; Febbraio, M.; Silverstein, R.; Savill, J.; Lacy-Hulbert, A. Requirements for apoptotic cell contact in regulation of macrophage responses. J. Immunol. 2006, 177, 4047–4054. [Google Scholar]
- Huynh, M.L.; Fadok, V.A.; Henson, P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 2002, 109, 41–50. [Google Scholar]
- van Zandbergen, G.; Bollinger, A.; Wenzel, A.; Kamhawi, S.; Voll, R.; Klinger, M.; Muller, A.; Holscher, C.; Herrmann, M.; Sacks, D.; Solbach, W.; Laskay, T. Leishmania disease development depends on the presence of apoptotic promastigotes in the virulent inoculum. Proc. Natl. Acad. Sci. USA 2006, 103, 13837–13842. [Google Scholar]
- Gaipl, U.S.; Kuhn, A.; Sheriff, A.; Munoz, L.E.; Franz, S.; Voll, R.E.; Kalden, J.R.; Herrmann, M. Clearance of apoptotic cells in human SLE. Curr. Dir. Autoimmun. 2006, 9, 173–187. [Google Scholar]
- Herrmann, M.; Voll, R.E.; Zoller, O.M.; Hagenhofer, M.; Ponner, B.B.; Kalden, J.R. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998, 41, 1241–1250. [Google Scholar] [CrossRef]
- Baumann, I.; Kolowos, W.; Voll, R.E.; Manger, B.; Gaipl, U.; Neuhuber, W.L.; Kirchner, T.; Kalden, J.R.; Herrmann, M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002, 46, 191–201. [Google Scholar] [CrossRef]
- Gaipl, U.S.; Munoz, L.E.; Grossmayer, G.; Lauber, K.; Franz, S.; Sarter, K.; Voll, R.E.; Winkler, T.; Kuhn, A.; Kalden, J.; Kern, P.; Herrmann, M. Clearance deficiency and systemic lupus erythematosus (SLE). J. Autoimmun. 2007, 28, 114–121. [Google Scholar] [CrossRef]
- Munoz, L.E.; Janko, C.; Grossmayer, G.E.; Frey, B.; Voll, R.E.; Kern, P.; Kalden, J.R.; Schett, G.; Fietkau, R.; Herrmann, M.; Gaipl, U.S. Remnants of secondarily necrotic cells fuel inflammation in systemic lupus erythematosus. Arthritis Rheum. 2009, 60, 1733–1742. [Google Scholar] [CrossRef]
- Fiedler, K.; Simons, K. Annexin homologues in Giardia lamblia. Trends Biochem. Sci. 1995, 20, 177–178. [Google Scholar] [CrossRef]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef]
- Probst-Cousin, S.; Berghoff, C.; Neundorfer, B.; Heuss, D. Annexin expression in inflammatory myopathies. Muscle Nerve 2004, 30, 102–110. [Google Scholar] [CrossRef]
- Huber, R.; Berendes, R.; Burger, A.; Schneider, M.; Karshikov, A.; Luecke, H.; Romisch, J.; Paques, E. Crystal and molecular structure of human annexin V after refinement. Implications for structure, membrane binding and ion channel formation of the annexin family of proteins. J. Mol. Biol. 1992, 223, 683–704. [Google Scholar] [CrossRef]
- Huber, R.; Romisch, J.; Paques, E.P. The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J. 1990, 9, 3867–3874. [Google Scholar]
- Huber, R.; Schneider, M.; Mayr, I.; Romisch, J.; Paques, E.P. The calcium binding sites in human annexin V by crystal structure analysis at 2.0 A resolution. Implications for membrane binding and calcium channel activity. FEBS Lett. 1990, 275, 15–21. [Google Scholar] [CrossRef]
- Koopman, G.; Reutelingsperger, C.P.; Kuijten, G.A.; Keehnen, R.M.; Pals, S.T.; van Oers, M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar]
- Krysko, D.V.; Vanden Berghe, T.; D'Herde, K.; Vandenabeele, P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 2008, 44, 205–221. [Google Scholar] [CrossRef]
- Stuart, M.C.; Reutelingsperger, C.P.; Frederik, P.M. Binding of annexin V to bilayers with various phospholipid compositions using glass beads in a flow cytometer. Cytometry 1998, 33, 414–419. [Google Scholar] [CrossRef]
- Reutelingsperger, C.P.; Hornstra, G.; Hemker, H.C. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur. J. Biochem. 1985, 151, 625–629. [Google Scholar] [CrossRef]
- Reutelingsperger, C.P.; Kop, J.M.; Hornstra, G.; Hemker, H.C. Purification and characterization of a novel protein from bovine aorta that inhibits coagulation. Inhibition of the phospholipid-dependent factor-Xa-catalyzed prothrombin activation, through a high-affinity binding of the anticoagulant to the phospholipids. Eur. J. Biochem. 1988, 173, 171–178. [Google Scholar] [CrossRef]
- Munoz, L.E.; Frey, B.; Pausch, F.; Baum, W.; Mueller, R.B.; Brachvogel, B.; Poschl, E.; Rodel, F.; von der Mark, K.; Herrmann, M.; Gaipl, U.S. The role of annexin A5 in the modulation of the immune response against dying and dead cells. Curr. Med. Chem. 2007, 14, 271–277. [Google Scholar] [CrossRef]
- Lane, D.A.; Philippou, H.; Huntington, J. A. Directing thrombin. Blood 2005, 106, 2605–2612. [Google Scholar] [CrossRef]
- Lord, S.T. Fibrinogen and fibrin: scaffold proteins in hemostasis. Curr. Opin. Hematol. 2007, 14, 236–241. [Google Scholar] [CrossRef]
- Monroe, D. M.; Hoffman, M.; Roberts, H. R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1381–1389. [Google Scholar]
- Van Heerde, W.L.; Sakariassen, K.S.; Hemker, H.C.; Sixma, J.J.; Reutelingsperger, C.P.; de Groot, P.G. Annexin V inhibits the procoagulant activity of matrices of TNF-stimulated endothelium under blood flow conditions. Arterioscler. Thromb. 1994, 14, 824–830. [Google Scholar] [CrossRef]
- Ravassa, S.; Bennaghmouch, A.; Kenis, H.; Lindhout, T.; Hackeng, T.; Narula, J.; Hofstra, L.; Reutelingsperger, C. Annexin A5 down-regulates surface expression of tissue factor: a novel mechanism of regulating the membrane receptor repertoir. J. Biol. Chem. 2005, 280, 6028–6035. [Google Scholar]
- Brachvogel, B.; Welzel, H.; Moch, H.; von der Mark, K.; Hofmann, C.; Poschl, E. Sequential expression of annexin A5 in the vasculature and skeletal elements during mouse development. Mech. Dev. 2001, 109, 389–393. [Google Scholar] [CrossRef]
- Munoz, L.E.; Franz, S.; Pausch, F.; Furnrohr, B.; Sheriff, A.; Vogt, B.; Kern, P. M.; Baum, W.; Stach, C.; von Laer, D.; Brachvogel, B.; Poschl, E.; Herrmann, M.; Gaipl, U.S. The influence on the immunomodulatory effects of dying and dead cells of Annexin V. J. Leukoc. Biol. 2007, 81, 6–14. [Google Scholar]
- Frey, B.; Munoz, L.E.; Pausch, F.; Sieber, R.; Franz, S.; Brachvogel, B.; Poschl, E.; Schneider, H.; Rodel, F.; Sauer, R.; Fietkau, R.; Herrmann, M.; Gaipl, U.S. The immune reaction against allogeneic necrotic cells is reduced in Annexin A5 knock out mice whose macrophages display an anti-inflammatory phenotype. J. Cell. Mol. Med. 2009, 13, 1391–1399. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Schildkopf, P.; Rodel, F.; Weiss, E.M.; Munoz, L.E.; Herrmann, M.; Fietkau, R.; Gaipl, U.S. AnnexinA5 renders dead tumor cells immunogenic-implications for multimodal cancer therapies. J. Immunotoxicol. 2009, 6, 209–216. [Google Scholar] [CrossRef]
- Stach, C.M.; Turnay, X.; Voll, R.E.; Kern, P.M.; Kolowos, W.; Beyer, T.D.; Kalden, J.R.; Herrmann, M. Treatment with annexin V increases immunogenicity of apoptotic human T-cells in Balb/c mice. Cell Death Differ. 2000, 7, 911–915. [Google Scholar] [CrossRef]
- Bondanza, A.; Zimmermann, V.S.; Rovere-Querini, P.; Turnay, J.; Dumitriu, I.E.; Stach, C.M.; Voll, R.E.; Gaipl, U. S.; Bertling, W.; Poschl, E.; Kalden, J.R.; Manfredi, A.A.; Herrmann, M. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J. Exp. Med. 2004, 200, 1157–1165. [Google Scholar] [CrossRef]
- Callahan, M.K.; Popernack, P.M.; Tsutsui, S.; Truong, L.; Schlegel, R.A.; Henderson, A.J. Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J. Immunol. 2003, 170, 4840–4845. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chaurio, R.A.; Janko, C.; Muñoz, L.E.; Frey, B.; Herrmann, M.; Gaipl, U.S. Phospholipids: Key Players in Apoptosis and Immune Regulation. Molecules 2009, 14, 4892-4914. https://doi.org/10.3390/molecules14124892
Chaurio RA, Janko C, Muñoz LE, Frey B, Herrmann M, Gaipl US. Phospholipids: Key Players in Apoptosis and Immune Regulation. Molecules. 2009; 14(12):4892-4914. https://doi.org/10.3390/molecules14124892
Chicago/Turabian StyleChaurio, Ricardo A., Christina Janko, Luis E. Muñoz, Benjamin Frey, Martin Herrmann, and Udo S. Gaipl. 2009. "Phospholipids: Key Players in Apoptosis and Immune Regulation" Molecules 14, no. 12: 4892-4914. https://doi.org/10.3390/molecules14124892
APA StyleChaurio, R. A., Janko, C., Muñoz, L. E., Frey, B., Herrmann, M., & Gaipl, U. S. (2009). Phospholipids: Key Players in Apoptosis and Immune Regulation. Molecules, 14(12), 4892-4914. https://doi.org/10.3390/molecules14124892