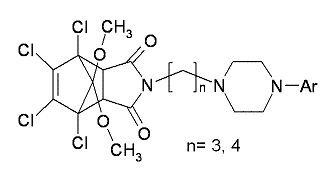
Synthesis and Evaluation of in Vitro Biological Activity of 4-Substituted Arylpiperazine Derivatives of 1,7,8,9-Tetrachloro-10,10-dimethoxy-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione
Abstract
:Introduction
Results and Discussion
Experimental
General
General procedure for the preparation of arylpiperazine derivatives 2a–2j and 3a–3j
General procedure for the preparation of hydrochloride salts of compounds 2a–2j and 3a–3j
X-Ray crystal structure analysis of 1 and 3b diethyl ether hemisolvate
Cell-based assays
Cytotoxicity Assays
Conclusions
References
- Tan, S.L.; Pause, A.; Shi, Y.; Sonenberg, N. Hepatitis C therapeutics: Current status and emerging strategies. Nat. Rev. Drug. Discov. 2002, 1, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Westaway, E.G. Flavivirus replication strategy. Adv. Virus Res. 1987, 33, 45–90. [Google Scholar] [PubMed]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, M.C. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Heinz, F.X. Flaviviruses. In Fields Virology, 3rd ed.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1996; Vol. 1, pp. 961–1034. [Google Scholar]
- Nettelton, P.E.; Entrican, G. Ruminant pestiviruses: A review. Br. Vet. J. 1995, 151, 615–642. [Google Scholar] [CrossRef]
- Rice, M.C. Flaviviridae: The Viruses and Their Replication. In Fields Virology, 3rd ed.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1996; Vol. 1, pp. 931–960. [Google Scholar]
- Wang, M.; Ng, K.K.S.; Cherney, M.M.; Chan, L.; Yannopoulos, C.G.; Bedard, J.; Morin, N.; Nguen-Ba, N.; Alaoui-Ismaili, M.H.; Bethell, R.C.; James, M.N.C. Non-nucleoside analogue inhibitors bind to an allosteric site on HCV NS5B polymerase. J. Biol. Chem. 2003, 278, 9489–9495. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, P.H.; Di Bisceglie, A.M. The progression of hepatitis B- and C-infections to chronic liver disease and hepatocellular carcinoma: Epidemiology and pathogenesis. Med. Clin. North. Am. 2005, 89, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Echevarria-Mayo, J.M. Etiology and pathogenesis of viral hepatitis. Enferm. Infecc. Microbiol. Clin. 2006, 24, 45–56. [Google Scholar] [PubMed]
- Bosch, F.X.; Ribes, J.; Cleries, R.; Diaz, M. Epidemiology of hepatocellular carcinoma. Clin. Liver Dis. 2005, 9, 191–211. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. Viruses and Human Disease; Academic Press. A Division of Harcourt, Inc.: London, UK, 2002. [Google Scholar]
- Wong, K.T. Enterovirus-associated neurological disease with special reference to enterovirus 71. Neurol. J. Southest Asia 2000, 5, 47–49. [Google Scholar]
- Chern, I.H.; Shia, K.S.; Shih, S.R.; Hsu, T.A.; Tai, C.L. Anti-enterovirus compounds. US Pat. 6815444, 2004. [Google Scholar]
- Chern, J.H.; Shia, K.S.; Hsu, T.A.; Tai, C.L.; Chung-chi, L.; Lee, C.C.; Lee, Y.C.; Chang, C.S.; Tseng, S.N.; Shih, S.R. Design, synthesis, and structure-activity relationships of pyrazolo[3,4-d]pyrimidines: A novel class of potent enterovirus inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Romero, D.L.; Morge, R.A.; Genin, M.J.; Biles, C.; Busso, M.; Resnick, L.; Althaus, I.W.; Reusser, F.; Thomas, R.C.; Tarpley, W.G. Bis(heteroaryl)piperazine (BHAP) reverse transcriptase inhibitors: structure-activity relationships of novel substituted indole analogues and the identification of 1-[(5-methanesulfonamido-1H-indol-2-yl)-carbonyl]-4-[3-[(1-methylethyl)-amino]-pyridinyl]piperazine monomethanesulfonate (U-90152S), a second-generation clinical candidate. J. Med. Chem. 1993, 36, 1505–1508. [Google Scholar] [PubMed]
- Gulick, R.M.; Su, Z.; Flexner, C.; Hughes, M.D.; Skolnik, P.R.; Wilkin, T.J.; Gross, R.; Krambrink, A.; Coakley, E.; Greaves, W.L.; Zolopa, A.; Reichman, R.; Godfrey, C.; Hirsch, M.; Kuritzkes, D.R. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J. Infect. Dis. 2007, 196, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Peri, C.A. New polychlorinated derivatives containing the ring of 1,4-endomethylenecyclohexane. Gazz. Chim. Ital. 1955, 85, 1118–1140. [Google Scholar]
- Sheldrick, G.M. Program for a crystal structure solution. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyster, J.; De Clercq, E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Meth. 1998, 20, 309–321. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1,2,3, 2a–2j and 3a–3j are available from authors. |


| Comp. | M.p. (°C) | Yield (%) | Formula | Analysis Calculated/Found | 1H-NMR, δH (J in Hz), solvent–CDCl3 | ||
|---|---|---|---|---|---|---|---|
| %C | %H | %N | |||||
| 2 | oil | 72 | C14H14Cl5N O4 | 36.32 36.23 | 3.25 3.58 | 2.82 3.09 | 3.64 (s, 3H, OCH3); 3.62 (s, 3H, OCH3); 3.44–3.37 (m, 6H, CH, CH2); 2.03–2.0 (m, 2H, CH2); 1.87–1.81 (m, 1H, CH2); 1.67–1.57 (m, 1H, CH2) |
| 3 | oil | 74 | C15H16BrCl4NO4 | 38.43 38.39 | 3.23 3.34 | 3.20 3.21 | 3.65 (s, 3H, OCH3); 3.63 (s, 2H, CH); 3.59 (s, 3H, OCH3); 3.60–3.59 (m, 2H, CH2); 3.53–3.50 (t, 2H, CH2, J = 6.4); 1.97–1.91 (t, 2H, CH2, J = 10.2) |
| 2a | 233 | 85 | C26H32Cl5N3O5 | 48.50 48.37 | 5.01 5.25 | 6.53 6.34 | 13.3 (s, 1H, HCl); 8.17–8.15 (d, 1H, CHarom., J = 8); 7.48–7.44 (t, 1H, CHarom., J = 8.6); 7.09–7.03 (m, 2H, CHarom.); 5.03–4.98 (m, 2H, CH2N); 4.35–4.33 (m, 2H, CH2N); 4.06 (s, 3H, OCH3); 3.73 (s, 2H, CH); 3.65 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.65–3.56 (m, 2H, CH2N); 3.23–3.2 (m, 4H, CH2N); 1.96–1.92 (m, 2H, CH2); 1.65–1.61 (m, 2H, CH2) |
| 2b | 256 | 86 | C23H28Cl5N5O4 | 44.86 44.67 | 4.58 4.87 | 11.37 11.13 | 13.04 (s, 1H, HCl); 8.36 (s, 2H, CHarom.); 6.64 (s, 1H, CHarom.); 4.89–4.85 (m, 2H, CH2N); 3.87–3.91 (m, 2H, CH2N); 3.70 (s, 2H, CH); 3.65 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.46–3.43 (m, 2H, CH2N); 3.0–2.99 (m, 2H, CH2N); 2.79–2.77 (m, 2H,CH2N); 1.96–1.92 (m, 2H, CH2); 1.60–1.58 (m, 2H, CH2) |
| 2c | 243 | 88 | C25H30Cl5N3O4 | 48.92 48.79 | 4.93 5.0 | 6.85 6.81 | 12.78 (s, 1H, HCl); 7.34–7.30 (s, 2H, CHarom.); 7.02–7.04 (s, 3H, CHarom.); 3.76 (s, 2H, CH); 3.65 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.65–3.56 (m, 6H, CH2N); 3.46–3.44 (m, 2H, CH2N); 3.07–2.0 (m, 2H,CH2N); 1.97–1.95 (m, 2H, CH2); 1.63–1.60 (m, 2H, CH2) |
| 2d | 248 | 76 | C24H29Cl5N4O4 | 46.89 46.78 | 4.75 4.95 | 9.11 9.02 | 13.01 (s, 1H, HCl); 8.01 (s, 2H, CHarom.); 7.13–7.06 (s, 1H, CHarom.); 4.67–4.60 (m, 2H, CH2N); 4.21–4.19 (m, 2H, CH2N); 3.73 (m, 2H, CH2N); 3.65 (s, 2H, CH); 3.65 (s, 3H, OCH3); 3.57 (s, 3H, OCH3); 3.27–3.15 (m, 4H, CH2N); 1.94–1.92 (m, 2H, CH2); 1.63–1.61 (m, 2H, CH2) |
| 2e | 206 | 79 | C25H29Cl5FN3O4 | 47.53 47.25 | 4.63 4.64 | 6.65 6.51 | 13.25 (s, 1H, HCl); 7.61–7.6 (m, 2H, CHarom.); 7.15–7.12 (m, 2H, CHarom.); 4.39–4.35 (m, 2H, CH2N); 3.85–3.83 (m, 2H, CH2N); 3.72 (s, 2H, CH); 3.65 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.65–3.56 (m, 4H, CH2N); 3.49–3.41 (m, 2H, CH2N); 1.95–1.92 (m, 2H, CH2); 1.65–1.63 (m, 2H, CH2). |
| 2f | 211 | 68 | C26H32Cl5N3O4 | 49.74 49.56 | 5.14 5.41 | 6.69 6.34 | 12.96 (s, 1H, HCl); 7.66–7.63 (m, 2H, CHarom.); 7.48–7.45 (m, 3H, CHarom.); 4.26–4.24 (m, 2H, CH2); 4.02–4.0 (m, 4H, CH2N); 3.72 (s, 2H, CH); 3.64 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.46–3.41 (m, 4H, CH2N); 3.16–3.14 (m, 2H, CH2N); 1.89–1.85 (m, 2H, CH2); 1.69–1.62 (m, 2H, CH2) |
| 2g | 230 | 87 | C25H29Cl6N3O4 | 46.32 46.26 | 4.51 4.81 | 6.48 6.68 | 13.23 (s, 1H, HCl); 7.58–7.56 (m, 2H, CHarom.); 7.44–7.42 (s, 2H, CHarom.); 4.44–4.38 (m, 2H, CH2N); 3.85–3.83 (m, 2H, CH2N); 3.72 (s, 2H, CH); 3.65 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.65–3.56 (m, 2H, CH2N); 3.50–3.47 (m, 2H, CH2N); 3.19–3.17 (m, 2H, CH2N); 1.95–1.92 (m, 2H, CH2); 1.65–1.63 (m, 2H, CH2) |
| 2h | 231 | 86 | C26H32Cl5N3O5 | 48.5 48.42 | 5.01 5.23 | 6.53 6.56 | 12.91 (s, 1H, HCl); 7.25–7.23 (m, 1H, CHarom.); 6.72–6.63 (s, 3H, CHarom.); 3.98–3.92 (m, 2H, CH2N); 3.80 (s, 3H, OCH3); 3.76 (s, 2H, CH); 3.65 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.65–3.56 (m, 4H, CH2N); 3.3–3.28 (m, 2H, CH2N); 1.96–1.92 (m, 2H, CH2); 1.63–1.6 (m, 2H, CH2) |
| 2i | 213 | 84 | C26H32Cl5N3O4 | 49.74 49.72 | 5.14 5.18 | 6.69 6.44 | 12.97 (s, 1H, HCl); 7.71–7.7 (m, 1H, CHarom.); 7.36–7.3 (s, 3H, CHarom.); 4.68–4.46 (m, 4H, CH2N); 3.83 (s, 2H, CH); 3.74–3.65 (m, 2H. CH2N); 3.65 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.50–3.48 (m, 2H, CH2N); 3.24–3.22 (m, 2H, CH2N); 2.74 (s, 3H, CH3); 1.96–1.924 (m, 2H, CH2); 1.66–1.63 (m, 2H, CH2) |
| 2j | 215 | 76 | C25H29Cl5FN3O4 | 47.53 47.49 | 4.63 4.78 | 6.65 6.55 | 12.72 (s, 1H, HCl); 7.1–6.96 (m, 4H, CHarom.); 4.77–4.74 (m, 2H, CH2N); 3.76 (s, 2H, CH); 3.72–3.65 (m, 2H, CH2N); 3.65 (s, 3H, OCH3); 3.58 (s, 3H, OCH3); 3.5–3.4 (m, 2H, CH2N); 3.06–3.05 (m, 2H, CH2N); 1.97–1.96 (m, 2H, CH2); 1.63–1.61 (m, 2H, CH2) |
| 3a | 185 | 87 | C25H30Cl5N3O5 | 50.61 50.60 | 4.93 4.91 | 7.08 7.09 | 7.02–6.99 (m, 1H, CHarom.); 6.93–6.91 (s, 2H, CHarom.); 6.89–6.85 (m, 1H, CHarom.); 3.86 (s, 3H, OCH3); 3.64 (s, 5H, CH, OCH3); 3.59 (s, 3H, OCH3); 3.54–3.52 (m, 2H, CH2N); 3.16–3.14 (m, 4H, CH2N); 2.74–2.7 (m, 4H, CH2N); 2.54–2.52–1.92 (m, 2H, CH2N); 1.78–1.76 (m, 2H, CH2) |
| 3b | 244 | 78 | C22H26Cl5N5O4 | 46.74 46.52 | 4.46 4.61 | 12.39 12.28 | 11.17 (s, 1H, HCl); 8.44–8.43 (d, 2H, CHarom., J = 4.8); 6.77–6.74 (t, 1H, CHarom., J = 4.6); 3.81 (m, 2H, CH2N); 3.60 (s, 2H, CH); 3.49–3.47 (m, 6H, CH2N); 3.41 (s, 6H, OCH3); 3.06–3.2.97 (m, 4H, CH2N); 1.87–1.83 (m, 2H, CH2) |
| 3c | 246 | 75 | C24H28Cl5N3O4 | 48.06 48.03 | 4.71 4.59 | 7.01 6.8 | 12.89 (s, 1H, HCl); 7.33–7.29 (m, 2H, CHarom.); 7.01–6.99 (m, 3H, CHarom.,); 3.74–3.72 (m, 6H, CH2N); 3.65 (s, 8H, CH, OCH3); 3.59–3.54 (m, 2H, CH2N); 3.5–2.45 (m, 4H, CH2N); 2.18–2.16 (m, 2H, CH2) |
| 3d | 248 | 78 | C23H27Cl5N4O4 | 45.98 45.58 | 4.53 4.94 | 9.33 9.02 | 13.04 (s, 1H, HCl); 8.21 (d, 1H, CHarom., J = 4); 7.6–7.58 (m, 2H, CHarom.); 6.8–6.77 (s, 2H, CHarom.); 4.34 (m, 2H, CH2N); 3.90–3.89 (m, 2H, CH2N); 3.8 (s, 2H CH); 3.65 (s, 6H, OCH3); 3.63–3.37 (m, 4H, CH2N);3.0–2.86 (m, 4H, CH2N); 2.17–2.16 (m, 2H, CH2) |
| 3e | 237 | 80 | C24H27Cl5FN3O4 | 46.66 46.22 | 4.41 4.73 | 6.80 6.51 | 12.83 (s, 1H, HCl); 7.09–7.03 (m, 4H, CHarom.); 3.76–3.45 (m, 8H, CH2N); 3.65 (s, 5H, CH, OCH3); 3.58 (s, 3H, OCH3); 3.15–3.1 (m, 4H, CH2N); 2.18–2.14 (m, 2H, CH2) |
| 3f | 241 | 64 | C25H30Cl5N3O4 | 48.92 48.72 | 4.93 4.85 | 6.85 6.72 | 7.64–6.61 (m, 2H, CHarom.); 7.47–7.45 (s, 3H, CHarom.); 4.23–4.21 (m, 2H, CH2); 3.69–3.67 (m, 4H, CH2N); 3.65–3.44 (m, 6H, CH2N); 3.65 (s, 5H, CH, OCH3); 3.57 (s, 3H, OCH3); 3.11–3.08 (m, 2H, CH2N); 2.03–2.0 (m, 2H, CH2) |
| 3g | 250 | 85 | C24H27Cl6N3O4 | 45.45 45.36 | 4.29 4.35 | 6.63 6.46 | 13.13 (s, 1H, HCl); 7.33–7.26 (m, 4H, CHarom.); 4.05–4.0 (m, 2H, CH2N); 3.74–3.71 (m, 2H, CH2N); 3.65 (s, 5H, CH, OCH3); 3.59 (s, 3H, OCH3); 3.65–3.58 (m, 4H, CH2N); 3.42–3.4 (m, 2H, CH2N); 3.14–3.12 (m, 2H, CH2N); 2.16–2.14 (m, 2H, CH2) |
| 3h | 219 | 81 | C25H30Cl5N3O5 | 47.68 47.82 | 4.80 5.04 | 6.67 6.52 | 12.9 (s, 1H, HCl); 7.23–7.21 (m, 1H, CHarom.); 6.68–6.59 (m, 3H, CHarom.); 3.9 (s, 3H, OCH3); 3.89–3.74 (m, 4H, CH2N); 3.65 (s, 5H, CH, OCH3); 3.58 (s, 3H, OCH3); 3.64–3.57 (m, 4H, CH2N); 3.18–3.1 (m, 4H, CH2N); 2.17–2.14 (m, 2H, CH2). |
| 3i | 229 | 75 | C25H30Cl5N3O4 | 48.92 48.82 | 4.93 5.13 | 6.85 6.75 | 12.83 (s, 1H, HCl); 7.16–7.12 (m, 1H, CHarom.); 6.34–6.32 (m, 3H, CHarom.); 3.93–4.0 (m, 2H, CH2N); 3.75–3.71 (m, 2H, CH2N); 3.65 (s, 5H, CH, OCH3); 3.59 (s, 3H, OCH3); 3.58–3.31 (m, 4H, CH2N); 3.15–3.12 (m, 2H, CH2N); 2.43–2.4 (m, 2H, CH2N); 2.17–2.14 (m, 2H, CH2) |
| 3j | 224 | 73 | C24H27Cl5FN3O4 | 46.66 46.70 | 4.41 4.66 | 6.80 6.57 | 12.92 (s, 1H, HCl); 7.08–7.04 (m, 4H, CHarom.); 3.74–3.72 (m, 2H, CH2N); 3.69–3.67 (m, 2H, CH2N); 3.65 (s, 5H, CH, OCH3); 3.58 (s, 3H, OCH3); 3.57–3.45 (m, 4H, CH2N); 3.08–3.04 (m, 4H, CH2N); 17–2.14 (m, 2H, CH2) |
| 1 | 3b | ||
|---|---|---|---|
| Empirical formula | C11H9Cl4NO4 | C22H26Cl5N5O4 × (C2H5O)0.5 | |
| Formula weight | 360.99 | 638.79 | |
| Crystal system, space group | triclinic, P-1 | monoclinic, P21/c | |
| Unit cell dimensions | a (Å) | 8.304(2) | 16.230(3) |
| b (Å) | 13.554(3) | 8.642(2) | |
| c (Å) | 14.642(3) | 22.153(4) | |
| α (°) | 111.97(3) | 90 | |
| β (°) | 96.28(3) | 104.03(3) | |
| γ (°) | 103.63(3) | 90 | |
| Volume (Å3) | 1449.2(6) | 3014.5(10) | |
| Z, Calculated density (g cm–3) | 4, 1.655 | 4, 1.408 | |
| F(000) | 728 | 1324 | |
| Absorption coefficient (mm-1) | 7.548 | 4.725 | |
| Crystal size (mm) | 0.32 × 0.25 × 0.18 | 0.42 × 0.19 × 0.11 | |
| Absorption corr.; T min, max | 0.054, 0.151 | 0.145, 0.245 | |
| θ range for data collection (°) | 5.6–72.9 | 5.5–75.8 | |
| Limiting indices | -10 ≤ h ≤ 0, -15 ≤ k ≤ 15, -16 ≤ l ≤ 17 | -20 ≤ h ≤ 19, 0 ≤ k ≤ 10, 0 ≤ l ≤ 27 | |
| Reflections collected / unique / observed [I > 2σ (I)] | 5960 / 5596 / 2773 [Rint = 0.0424] | 6295 / 6162 / 2021 [Rint = 0.0354] | |
| Data / parameters | 5596 / 366 | 6162 / 365 | |
| Goodness-of-fit on F2 | 1.074 | 0.944 | |
| Final R indices [I > 2σ (I)] | R1 = 0.0452, wR2 = 0.1370 | R1 = 0.0620, wR2 = 0.1515 | |
| Max and min Δρ (e Å-3) | 0.43 and -0.32 | 0.59 and -0.39 | |
| Extinction coeff. | 0.0018(3) | 0.0019(3) | |
| CCDC No | 713452 | 713453 | |
| Compd. | aMT-4 CC50 [µM] | bHIV-1 EC50 [µM] | cMDBK CC50 [µM] | dBFDV EC50 [µM] | eBHK-21 CC50 [µM] | fYFV EC50 [µM] | fReo-1 EC50 [µM] |
|---|---|---|---|---|---|---|---|
| 1 | >100 | >100 | - | - | - | - | - |
| 2a | 24 | >24 | 86 | >86 | 13 | >13 | >13 |
| 2b | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 2c | 45 | >45 | >100 | >100 | >100 | >100 | >100 |
| 2d | 36 | >36 | >100 | >100 | >100 | >100 | >100 |
| 2e | 16 | >16 | >100 | >100 | 64 | >64 | >64 |
| 2g | 11 | >11 | >100 | >100 | >100 | >100 | >100 |
| 2h | 18 | >18 | >65 | >65 | 11 | >11 | >11 |
| 2i | 17 | >17 | >14 | >14 | 7 | >7 | >7 |
| 2j | 24 | >24 | >100 | >100 | 30 | >30 | >30 |
| 3a | 32 | >32 | 73 | >73 | 22 | >22 | >22 |
| 3c | 30 | >30 | 100 | >100 | 29 | >29 | >29 |
| 3d | 53 | >53 | >100 | >100 | >100 | >100 | >100 |
| 3e | 34 | >34 | 89 | >89 | 36 | >36 | >36 |
| 3i | 20 | >20 | 24 | >24 | 9 | >9 | >9 |
| Compd. | gVero-76 CC50 [µM] | hHSV-1 EC50 [µM] | hVV EC50 [µM] | hVSV EC50 [µM] | hCVB-2 EC50 [µM] | hSB-1 EC50 [µM] | |
| 1 | - | - | - | - | - | - | |
| 2a | 80 | >80 | >80 | >80 | >80 | >80 | |
| 2b | 95 | >95 | >95 | >95 | >95 | >95 | |
| 2c | 84 | >84 | >84 | >84 | 13 | >84 | |
| 2d | 95 | >95 | >95 | >95 | >95 | >95 | |
| 2e | 60 | >60 | >60 | >60 | >60 | >60 | |
| 2g | 95 | >95 | >95 | >95 | 10 | >95 | |
| 2h | 70 | >70 | >70 | >70 | >70 | >70 | |
| 2i | 45 | >45 | >45 | >45 | >45 | >45 | |
| 2j | 52 | >52 | >52 | >52 | >52 | >52 | |
| 3a | 60 | >60 | >60 | >60 | >60 | >60 | |
| 3c | 70 | >70 | >70 | >70 | >70 | >70 | |
| 3d | 82 | >82 | >82 | >82 | 17 | >82 | |
| 3e | 75 | >75 | >75 | >75 | >75 | >75 | |
| 3i | 34 | >34 | >34 | >34 | >34 | >34 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kossakowski, J.; Pakosinska-Parys, M.; Struga, M.; Dybala, I.; Koziol, A.E.; La Colla, P.; Marongiu, L.E.; Ibba, C.; Collu, D.; Loddo, R. Synthesis and Evaluation of in Vitro Biological Activity of 4-Substituted Arylpiperazine Derivatives of 1,7,8,9-Tetrachloro-10,10-dimethoxy-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. Molecules 2009, 14, 5189-5202. https://doi.org/10.3390/molecules14125189
Kossakowski J, Pakosinska-Parys M, Struga M, Dybala I, Koziol AE, La Colla P, Marongiu LE, Ibba C, Collu D, Loddo R. Synthesis and Evaluation of in Vitro Biological Activity of 4-Substituted Arylpiperazine Derivatives of 1,7,8,9-Tetrachloro-10,10-dimethoxy-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. Molecules. 2009; 14(12):5189-5202. https://doi.org/10.3390/molecules14125189
Chicago/Turabian StyleKossakowski, Jerzy, Magdalena Pakosinska-Parys, Marta Struga, Izabela Dybala, Anna E. Koziol, Paolo La Colla, Laura Ester Marongiu, Cristina Ibba, David Collu, and Roberta Loddo. 2009. "Synthesis and Evaluation of in Vitro Biological Activity of 4-Substituted Arylpiperazine Derivatives of 1,7,8,9-Tetrachloro-10,10-dimethoxy-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione" Molecules 14, no. 12: 5189-5202. https://doi.org/10.3390/molecules14125189
APA StyleKossakowski, J., Pakosinska-Parys, M., Struga, M., Dybala, I., Koziol, A. E., La Colla, P., Marongiu, L. E., Ibba, C., Collu, D., & Loddo, R. (2009). Synthesis and Evaluation of in Vitro Biological Activity of 4-Substituted Arylpiperazine Derivatives of 1,7,8,9-Tetrachloro-10,10-dimethoxy-4-azatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione. Molecules, 14(12), 5189-5202. https://doi.org/10.3390/molecules14125189