Wet Aerobic Oxidation of Lignin into Aromatic Aldehydes Catalysed by a Perovskite-type Oxide: LaFe1-xCuxO3 (x=0, 0.1, 0.2)
Abstract
:1. Introduction
2. Result and Discussion
2.1. Catalyst characterization
2.1.1. Surface oxygen species and Fe ion oxidation states
2.1.2. TPR profile of LaFe1-xCuxO3 (x=0, 0.1, 0.2)
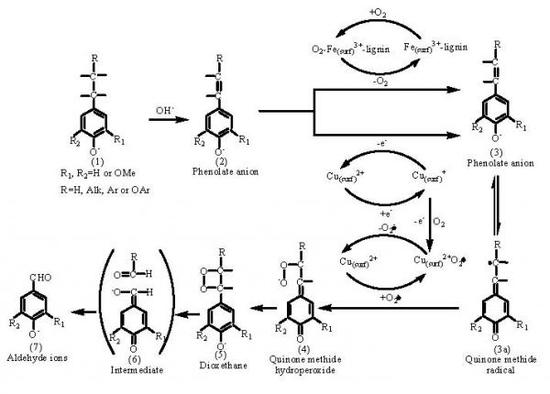
2.2. Catalytic oxidation lignin into aromatic aldehydes
3. Experimental
3.1. Lignin and catalyst preparation
3.2. Catalyst characterization [20]
3.3. Catalytic experiments [20]
4. Conclusions
Acknowledgements
References
- Rostrup-Nielsen, J.R. Making fuels from biomass. Science 2005, 308, 1421–1422. [Google Scholar] [CrossRef] [PubMed]
- Perlack, R.D.; Wright, L.L.; Turhollow, A.; Graham, R.L.; Stokes, B.; Erbach, D.C. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply; Oak Ridge National Laboratory 2005, DOE/GO-10295-2135. http://www.osti.gov/bridge.
- Juben, N.C.; James, A.D. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today 2007, 12, 59–70. [Google Scholar]
- Gaspar, A.; Evtuguin, D.V.; Pascoal, N.C. Oxygen bleaching of kraft pulp catalysed by Mn(III)-substituted polyoxometalates. Appl. Catal. A: Gen. 2003, 239, 157–168. [Google Scholar] [CrossRef]
- Sridhar, P.; Araujo, J.D.; Rodrigues, A.E. Modeling of Vanillaldehyde production in a structured bubble column reactor. Catal. Today 2005, 105, 574–581. [Google Scholar] [CrossRef]
- Sales, F.G.; Maranhão, L.C.A.; Filho, N.M.L.; Abreu, C.A.M. Kinetic Evaluation and Modeling of Lignin Catalytic Wet Oxidation to Selective Production of Aromatic Aldehydes. Ind. Eng. Chem. Res. 2006, 45, 6627–6631. [Google Scholar] [CrossRef]
- Sales, F.G.; Maranhão, L.C.A.; Filho, N.M.L.; Abreu, C.A.M. Experimental evaluation and continuous catalytic process for fine aldehyde production from lignin. Chem. Eng. Sci. 2007, 62, 5386–5391. [Google Scholar] [CrossRef]
- Mathias, A.L.; Rodrigues, A.E. Production of Vanillin by Oxidation of Pine Kraft Lignins with Oxygen. Holzforscung 1995, 49, 273–278. [Google Scholar] [CrossRef]
- Wu, G.X.; Heitz, M.; Chornet, E. Improved Alkaline Oxidation Process for the Production of Aldehydes (Vanillaldehyde and Syringaldehyde) from Steam-Explosion Hardwood Lignin. Ind. Eng. Chem. Res. 1994, 33, 718–723. [Google Scholar] [CrossRef]
- Wu, G.X.; Heitz, M. Catalytic mechanism of Cu2+ and Fe3+ in alkaline O2 Oxidation of Lignin. J. Wood. Chem. Technol. 1995, 15, 189–202. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Petukhov, D.V.; Selyutin, G.E. New Mechanism for the Catalytic Oxidation of Lignin to Vanillaldehyde. Kinet. Catal. 2004, 45, 603–611. [Google Scholar] [CrossRef]
- Villar, J.C.; Caperos, A.; García-Ochoa, F. Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant. Wood Sci. Technol. 2001, 35, 245–255. [Google Scholar] [CrossRef]
- Seiyama, T.; Tejuca, L.G.; Fierro, J.L.G. Properties and Applications of Perovskite-type Oxides; Marcel Dekker: New York, NY, USA, 1993; p. 215. [Google Scholar]
- Nitadori, T.; Ichiki, T.; Misono, M. Catalytic properties of perovskite-type mixed oxides (ABO3) consisting of rare earth and 3d transition metals. Bull. Chem. Soc. Jpn. 1988, 61, 621. [Google Scholar] [CrossRef]
- Szabo, V.; Bassir, M.; Van, N.A. Perovskite-type oxides synthesized by reactive grinding Part II: Catalytic properties of LaCo1−xFexO3 in VOC oxidation. Appl. Catal. B 2002, 37, 175–180. [Google Scholar] [CrossRef]
- Zhang, R.D.; Villanueva, A.; Alamdari, H. SCR of NO by propene over nano scale LaMn1-xCuxO3 perovskites. Appl. Catal. A 2006, 307, 85–97. [Google Scholar] [CrossRef]
- Yang, M.; Xu, A.H.; Du, H.Z. Removal of salicylic acid on perovskite-type oxide LaFeO3 catalyst in catalytic wet air oxidation process. J. Hazard. Mater. 2007, 139, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Levasseur, B.; Alamdari, H.; Kaliaguine, S. Mechanism of stearic acid oxidation over nanocrystalline La1−xAxBO3 (A = Sr, Ce; B = Co, Mn): The role of oxygen mobility. Appl. Catal. B 2008, 80, 51–61. [Google Scholar] [CrossRef]
- Bentivengaa, G.; Boninib, C.; Auriab, M.D. Degradation of steam-exploded lignin from beech by using Fenton’s reagent. Biomass Bioenerg. 2003, 24, 233–238. [Google Scholar] [CrossRef]
- Deng, H.B.; Lin, L.; Sun, Y.; Pang, C.S.; Zhuang, J.P.; Ouyang, P.K.; Li, J.Z.; Liu, S.J. Perovskite-type oxide lamno3: an Efficient and recyclable heterogeneous catalyst for the wet aerobic oxidation of lignin to aromatic aldehydes. Catal. Lett. 2008, 126, 106–111. [Google Scholar] [CrossRef]
- Fortuny, A.; Bengoa, C.; Font, J. Bimetallic catalysts for continuous catalytic wet air oxidation of phenol. J. Hazard. Mater. 1999, 64, 181–193. [Google Scholar] [CrossRef]
- Xu, A.H.; Yang, M.; Du, H.; Sun, C.L. Influence of partial replacement of Cu by Fe on the CWO of phenol in the Cu0.5-xFexZn0.5Al2O4 spinel catalysts. Catal.Commu. 2006, 7, 513–517. [Google Scholar] [CrossRef]
- De, L.C.; Goi, D.; Primavera, A. Wet oxidation of acetic acid catalyzed by doped ceria. Appl. Catal. B 1996, 11, 29–35. [Google Scholar]
- Carley, A.F.; Roberts, M.W.; Santra, A.K. interaction of oxygen and carbon monoxide with csau surfaces. J. Phys. Chem. B 1997, 101, 9978–9983. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Tejuca, L.G. Non-stoichiometric surface behaviour of LaMO3 oxides as evidenced by XPS. Appl. Surf. Sci. 1987, 27, 453–457. [Google Scholar] [CrossRef]
- Zhang, R.D.; Villanueva, A.; Alamdari, H. Fe-based perovskites substituted by copper and palladium for NO+CO reaction. J. Catal. 2006, 242, 241–253. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.; Dong, B.; Li, H.L. Controlled synthesis of highly ordered LaFeO3 nanowires using a citrate-based sol–gel route. Mater. Res. Bull. 2006, 41, 274–281. [Google Scholar] [CrossRef]
- Thomas, M.N.R.; Shiju, K.; Sreekumar, B.S. Cu–Co Synergism in Cu1−xCoxFe2O4 Catalysis and XPS Aspects. J. Catal. 2002, 210, 405–417. [Google Scholar]
- Arena, F.; Italiano, G.; Barbera, K. Solid-state interactions, adsorption sites and functionality of Cu-ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH. Appl. Catal. A 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Djinović, P.; Batista, J.; Pintar, A. Calcination temperature and CuO loading dependence on CuO-CeO2 catalyst activity for water-gas shift reaction. Appl. Catal. A 2008, 347, 23–33. [Google Scholar] [CrossRef]
- Ali, N.P.; Seyed, M.K.S.; Hamid, R.B. Effect of Mg, La and Ca promoters on the structure and catalytic behavior of iron-based catalysts in Fischer–Tropsch synthesis. Appl. Catal. A 2008, 348, 201–208. [Google Scholar]
- Barnabé, A.; Gaudon, M.; Bernard, C. Low temperature synthesis and structural characterization of over-stoichiometric LaMnO3+δ perovskites. Mater. Res. Bull. 2004, 39, 725–735. [Google Scholar] [CrossRef]
- Makshina, E.V.; Sirotin, S.V.; Berg, M.W.E. Characterization and catalytic properties of nanosized cobaltate particles prepared by in situ synthesis inside mesoporous molecular sieves. Appl. Catal. A-Gen. 2006, 312, 59–66. [Google Scholar] [CrossRef]
- Žabková, M.; Borges, E.A.S.; Rodrigues, A.E. Recovery of Vanillaldehyde from lignin/Vanillaldehyde mixture by using tubular ceramic ultrafiltration membranes. J. Membr. Sci. 2007, 301, 221–237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |








© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, J.; Deng, H.; Lin, L. Wet Aerobic Oxidation of Lignin into Aromatic Aldehydes Catalysed by a Perovskite-type Oxide: LaFe1-xCuxO3 (x=0, 0.1, 0.2). Molecules 2009, 14, 2747-2757. https://doi.org/10.3390/molecules14082747
Zhang J, Deng H, Lin L. Wet Aerobic Oxidation of Lignin into Aromatic Aldehydes Catalysed by a Perovskite-type Oxide: LaFe1-xCuxO3 (x=0, 0.1, 0.2). Molecules. 2009; 14(8):2747-2757. https://doi.org/10.3390/molecules14082747
Chicago/Turabian StyleZhang, Junhua, Haibo Deng, and Lu Lin. 2009. "Wet Aerobic Oxidation of Lignin into Aromatic Aldehydes Catalysed by a Perovskite-type Oxide: LaFe1-xCuxO3 (x=0, 0.1, 0.2)" Molecules 14, no. 8: 2747-2757. https://doi.org/10.3390/molecules14082747
APA StyleZhang, J., Deng, H., & Lin, L. (2009). Wet Aerobic Oxidation of Lignin into Aromatic Aldehydes Catalysed by a Perovskite-type Oxide: LaFe1-xCuxO3 (x=0, 0.1, 0.2). Molecules, 14(8), 2747-2757. https://doi.org/10.3390/molecules14082747