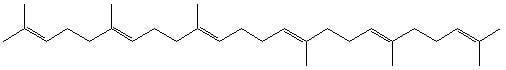
Squalene Emulsions for Parenteral Vaccine and Drug Delivery
Abstract
:1. Introduction to Squalene and Emulsions


2. Vaccines


3. Drug Delivery
 |

 |
 |
 |
4. Safety
5. Squalene and Emulsion Characterization

6. Conclusions
List of Abbreviations
| CAD | charged aerosol detector |
| CpG | oligonucleotide adjuvant containing cytosine-phosphate-guanine sequences |
| CWS | bacterial cell wall skeleton |
| DSC | differential scanning calorimetry |
| ESA | Experimental Squalene Adjuvant |
| FCA | Freund’s Complete Adjuvant |
| GWS | Gulf War Syndrome |
| HLB | hydrophilic-lipophilic balance |
| IFN-γ | interferon-γ |
| MPL | monophosphoryl lipid A |
| MTP-PE | muramyl tripeptide phosphatidyl ethanolamine |
| QS21 | quillaja saponin 21 |
| RAS | Ribi Adjuvant System |
| SAF | Syntex Adjuvant Formulation |
| SE | stable emulsion |
Acknowledgements
Potential Conflict of Interest
- Sample Availability: Squalene can be obtained from various chemical suppliers, including Sigma-Aldrich, Alfa Aesar, Acros Organics, and Wilshire Technologies. For specific squalene emulsion products described in this article, the corresponding suppliers should be contacted to determine availability.
References and Notes
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; Tanchuk, V.Y.; Prokopenko, V.V. Virtual computational chemistry laboratory - design and description. J. Comput. Aid. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- Whittenton, J.; Harendra, S.; Pitchumani, R.; Mohanty, K.; Vipulanandan, C.; Thevananther, S. Evaluation of asymmetric liposomal nanoparticles for encapsulation of polynucleotides. Langmuir 2008, 24, 8533–8540. [Google Scholar] [CrossRef]
- Chung, H.; Kim, T.W.; Kwon, M.; Kwon, I.C.; Jeong, S.Y. Oil components modulate physical characteristics and function of the natural oil emulsions as drug or gene delivery system. J. Control. Release 2001, 71, 339–350. [Google Scholar]
- Vogel, F.R.; Powell, M.F. A compendium of vaccine adjuvants and excipients. In Vaccine Design: The Subunit and Adjuvant Approach; Powell, M.F., Newman, M.J., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 141–228. [Google Scholar]
- Ernst, J.; Sheldrick, W.S.; Fuhrhop, J.H. The crystal structure of squalene. Angew. Chem. Int. Ed. 1976, 15, 778. [Google Scholar]
- Channon, H.J. The biological significance of the unsaponifiable matter of oils: Experiments with the unsaturated hydrocarbon, squalene (spinacene). Biochem. J. 1926, 20, 400–408. [Google Scholar]
- Tsujimoto, M. A highly unsaturated hydrocarbon in shark liver oil. Indust. Eng. Chem. 1916, 8, 889–896. [Google Scholar] [CrossRef]
- Thorbjarnarson, T.; Drummond, J.C. Occurrence of an unsaturated hydrocarbon in olive oil. Analyst 1935, 60, 23–29. [Google Scholar] [CrossRef]
- Alam, S.Q.; Brossard, J.; Mackinney, G. Detection and estimation of squalene in leaves. Nature 1962, 194, 479–480. [Google Scholar] [CrossRef]
- Murkovic, M.; Lechner, S.; Pietzka, A.; Bratacos, M.; Katzogiannos, E. Analysis of minor components in olive oil. J. Biochem. Biophys. Methods 2004, 61, 155–160. [Google Scholar] [CrossRef]
- Ollivier, D.; Artaud, J.; Pinatel, C.; Durbec, J.P.; Guerere, M. Triacylglycerol and fatty acid compositions of French virgin olive oils. Characterization by chemometrics. J. Agricul. Food Chem. 2003, 51, 5723–5731. [Google Scholar]
- Smith, T.J. Squalene: Potential chemopreventive agent. Expert Opin. Investig. Drugs 2000, 9, 1841–1848. [Google Scholar] [CrossRef]
- Deiana, M.; Corongui, F.P.; Dessi, M.A.; Scano, P.; Casu, M.; Lai, A. NMR determination of site-specific deuterium distribution (SNIF-NMR) in squalene from different sources. Magn. Reson. Chem. 2001, 39, 29–32. [Google Scholar] [CrossRef]
- Kelly, G.S. Squalene and its potential clinical uses. Alter. Med. Rev. 1999, 4, 29–36. [Google Scholar]
- Melnik, B.C.; Hollmann, J.; Erler, E.; Verhoeven, B.; Plewig, G. Microanalytical screening of all major stratum corneum lipids by sequential high-performance thin-layer chromatography. J. Invest. Dermatol. 1989, 92, 231–234. [Google Scholar]
- Robosky, L.C.; Wade, K.; Woolson, D.; Baker, J.D.; Manning, M.L.; Gage, D.A.; Reily, M.D. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J. Lipid Res. 2008, 49, 686–692. [Google Scholar] [CrossRef]
- Rozner, S.; Verkhovski, L.; Nissimov, Y.; Aserin, A.; Vilensky, R.; Danino, D.; Zouboulis, C.C.; Milner, Y.; Garti, N. Inhibition of cholesterol transport into skin cells in cultures by phytosterol-loaded microemulsion. Chem. Phys. Lipids 2008, 153, 109–118. [Google Scholar]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar]
- Walker, T.I. Can shark resources be harvested sustainably? A question revisited with a review of shark fisheries. Marine Freshwater Res. 1998, 49, 553–572. [Google Scholar] [CrossRef]
- Jenck, J.F.; Agterberg, F.; Droescher, M.J. Products and processes for a sustainable chemical industry: A review of achievements and prospects. Green Chem. 2004, 6, 544–556. [Google Scholar] [CrossRef]
- Stavroulias, S.; Panayiotou, C. Determination of optimum conditions for the extraction of squalene from olive pomace with supercritical CO2. Chem. Biochem. Eng. Quart. 2005, 19, 373–381. [Google Scholar]
- Dicker, D.W.; Whiting, M.C. Synthetical studies on terpenoids. Part I. The synthesis of squalene. J. Chem. Soc. 1958, 1994–2000. [Google Scholar]
- Zauner, W.; Lingnau, K.; Mattner, F.; von Gabain, A.; Buschle, M. Defined synthetic vaccines. Biol. Chem. 2001, 382, 581–595. [Google Scholar]
- Sesardic, D.; Dobbelaer, R. European union regulatory developments for new vaccine adjuvants and delivery systems. Vaccine 2004, 22, 2452–2456. [Google Scholar]
- Annual review of cosmetic ingredient safety assessments--2001/2002. Int. J. Toxicol. 2003, 22, 1–35.
- Komesvarakul, N.; Sanders, M.D.; Szekeres, E.; Acosta, E.J.; Faller, J.F.; Mentlik, T.; Fisher, L.B.; Nicoll, G.; Sabatini, D.A.; Scamehorn, J.F. Microemulsions of triglyceride-based oils: The effect of co-oil and salinity on phase diagrams. J. Cosmet. Sci. 2006, 57, 309–325. [Google Scholar]
- Vidal-Escales, E.; Borros, S. New methodology to follow the evolution of squalene by-products during model compound vulcanization studies. Talanta 2004, 62, 539–547. [Google Scholar] [CrossRef]
- Datta, R.N.; Hofstraat, J.W.; Geurts, F.A.J.; Talma, A.G. Fourier transform Raman spectroscopy for characterization of natural rubber reversion and of antireversion agents. Rubber Chem. Tech. 1999, 72, 829–843. [Google Scholar]
- Acosta, E.J.; Nguyen, T.; Witthayapanyanon, A.; Harwell, J.H.; Sabatini, D.A. Linker-based bio-compatible microemulsions. Environ. Sci. Technol. 2005, 39, 1275–1282. [Google Scholar] [CrossRef]
- Rastrelli, L.; Passi, S.; Ippolito, F.; Vacca, G.; De Simone, F. Rate of degradation of a-tocopherol, squalene, phenolics, and polyunsaturated fatty acids in olive oil during different storage conditions. J. Agricul. Food Chem. 2002, 50, 5566–5570. [Google Scholar] [CrossRef]
- Ketomaki, A.; Gylling, H.; Miettinen, T.A. Removal of intravenous Intralipid in patients with familial hypercholesterolemia during inhibition of cholesterol absorption and synthesis. Clin. Chim. Acta 2004, 344, 83–93. [Google Scholar]
- Lidgate, D.M.; Byars, N.E. Development of an emulsion-based muramyl dipeptide adjuvant formulation for vaccines. In Vaccine Design: The Subunit and Adjuvant Approach; Powell, M.F., Newman, M.J., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 313–324. [Google Scholar]
- Allison, A.C. The mode of action of immunological adjuvants. Dev. Biol. Stand. 1998, 92, 3–11. [Google Scholar]
- Wang, J.J.; Sung, K.C.; Yeh, C.H.; Fang, J.Y. The delivery and antinociceptive effects of morphine and its ester prodrugs from lipid emulsions. Int. J. Pharm. 2008, 353, 95–104. [Google Scholar] [CrossRef]
- Allison, A.C. Squalene and squalane emulsions as adjuvants. Methods 1999, 19, 87–93. [Google Scholar] [CrossRef]
- Baldridge, J.R.; Crane, R.T. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods 1999, 19, 103–107. [Google Scholar] [CrossRef]
- Foged, C.; Brodin, B.; Frokjaer, S.; Sundblad, A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005, 298, 315–322. [Google Scholar]
- Ryman, B.E.; Jewkes, R.F.; Jeyasingh, K.; Osborne, M.P.; Patel, H.M.; Richardson, V.J.; Tattersall, M.H.N.; Tyrrell, D.A. Potential applications of liposomes to therapy. Annals NY Acad. Sci. 1978, 308, 281–307. [Google Scholar]
- Mosca, F.; Tritto, E.; Muzzi, A.; Monaci, E.; Bagnoli, F.; Iavarone, C.; O’Hagan, D.; Rappuoli, R.; de Gregorio, E. Molecular and cellular signatures of human vaccine adjuvants. Proc. Nat. Acad. Sci. USA 2008, 105, 10501–10506. [Google Scholar]
- Seubert, A.; Monaci, E.; Pizza, M.; O'Hagan, D.T.; Wack, A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J. Immunol. 2008, 180, 5402–5412. [Google Scholar]
- Hamilton, J.A.; Byrne, R.; Whitty, G. Particulate adjuvants can induce macrophage survival, DNA synthesis, and a synergistic proliferative response to GM-CSF and CSF-1. J. Leukoc. Biol. 2000, 67, 226–232. [Google Scholar]
- Berg, J.C. An Introduction to Surfaces, Colloids and Nanoscience; University of Washington: Seattle, WA, USA, 2007. [Google Scholar]
- Mollet, H.; Grubenmann, A. Formulation Technology: Emulsions, Suspensions, Solid Forms; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Stone, H.D.; Xie, Z.X. Efficacy of experimental Newcastle disease water-in-oil oil-emulsion vaccines formulated from squalane and squalene. Avian Dis. 1990, 34, 979–983. [Google Scholar] [CrossRef]
- Yang, Y.W.; Shen, S.S. Enchanced antigen delivery via cell death induced by the vaccine adjuvants. Vaccine 2007, 25, 7763–7772. [Google Scholar] [CrossRef]
- Yang, Y.W.; Wei, A.C.; Shen, S.S. The immunogenicity-enhancing effect of emulsion vaccine adjuvants is independent of the dispersion type and antigen release rate--a revisit of the role of the hydrophile-lipophile balance (HLB) value. Vaccine 2005, 23, 2665–2675. [Google Scholar] [CrossRef]
- Yang, Y.W.; Wu, C.A.; Morrow, W.J.W. Cell death induced by vaccine adjuvants containing surfactants. Vaccine 2004, 22, 1524–1536. [Google Scholar]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Coll. Inter. Sci. 2004, 108-109, 303–318. [Google Scholar] [CrossRef]
- Al-Edresi, S.; Baie, S. Formulation and stability of whitening VCO-in-water nano-cream. Int. J. Pharm. 2009, 373, 174–178. [Google Scholar] [CrossRef]
- Bolland, J.L.; Hughes, H. The primary thermal oxidation product of squalene. J. Chem. Soc. 1949, 26, 492–497. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Ott, G.; Barchfeld, G.L.; Chernoff, D.; Radhakrishnan, R.; van Hoogevest, P.; van Nest, G. MF59: design and evaluation of a safe and potent adjuvant for human vaccines. In Vaccine Design: The Subunit and Adjuvant Approach; Powell, M.F., Newman, M.J., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 277–296. [Google Scholar]
- Carelli, C.; Audibert, F.; Chedid, L. Persistent enhancement of cell-mediated and antibody immune responses after administration of muramyl dipeptide derivatives with antigen in metabolizable oil. Infect. Immun. 1981, 33, 312–314. [Google Scholar]
- Allison, A.C.; Byars, N.E. Syntex adjuvant formulation. Res. Immunol. 1992, 143, 519–525. [Google Scholar] [CrossRef]
- Lidgate, D.M.; Fu, R.C.; Byars, N.E.; Foster, L.C.; Fleitman, J.S. Formulation of vaccine adjuvant muramyldipeptides. 3. Processing optimization, characterization, and bioactivity of an emulsion vehicle. Pharm. Res. 1989, 6, 748–752. [Google Scholar] [CrossRef]
- Lidgate, D.M.; Trattner, T.; Shultz, R.M.; Maskiewicz, R. Sterile filtration of a parenteral emulsion. Pharm. Res. 1992, 9, 860–863. [Google Scholar] [CrossRef]
- Allison, A.C.; Byars, N.E. An adjuvant formulation that selectively elicits the formation of antibodies of protective isotypes and of cell-mediated immunity. J. Immunol. Methods 1986, 95, 157–168. [Google Scholar] [CrossRef]
- Hsu, F.J.; Caspar, C.B.; Czerwinski, D.; Kwak, L.W.; Liles, T.M.; Syrengelas, A.; Taidi-Laskowski, B.; Levy, R. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma--long-term results of a clinical trial. Blood 1997, 89, 3129–3135. [Google Scholar]
- Kenney, R.T.; Edelman, R. Adjuvants for the future. In New Generation Vaccines; Levine, M.M., Kaper, J.B., Eds.; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- O'Hagan, D.T.; Singh, M. MF59: a safe and potent oil-in-water emulsion adjuvan. In Vaccine Adjuvants and Delivery Systems; Singh, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 115–129. [Google Scholar]
- Ott, G.; Singh, M.; Kazzaz, J.; Briones, M.; Soenawan, E.; Ugozzoli, M.; O’Hagan, D.T. A cationic sub-micron emulsion (MF59/DOTAP) is an effective delivery system for DNA vaccines. J. Control. Rel. 2002, 79, 1–5. [Google Scholar]
- Schultze, V.; D'Agosto, V.; Wack, A.; Novicki, D.; Zorn, J.; Hennig, R. Safety of MF59 adjuvant. Vaccine 2008, 26, 3209–3222. [Google Scholar] [CrossRef]
- Dupuis, M.; McDonald, D.M.; Ott, G. Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine 1999, 18, 434–439. [Google Scholar]
- Dupuis, M.; Murphy, T.J.; Higgins, D.; Ugozzoli, M.; van Nest, G.; Ott, G.; McDonald, D.M. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell. Immunol. 1998, 186, 18–27. [Google Scholar] [CrossRef]
- Baldridge, J.R.; Ward, J.R. Effective adjuvants for the induction of antigen-specific delayed-type hypersensitivity. Vaccine 1997, 15, 395–401. [Google Scholar] [CrossRef]
- Ott, G.; van Nest, G. Development of vaccine adjuvants: a historical perspective. In Vaccine Adjuvants and Delivery Systems; Singh, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 1–31. [Google Scholar]
- Blom, A.G.; Hilgers, L.A. Sucrose fatty acid sulphate esters as novel vaccine adjuvants: effect of the chemical composition. Vaccine 2004, 23, 743–754. [Google Scholar] [CrossRef]
- Sondak, V.K.; Sosman, J.A. Results of clinical trials with an allogeneic melanoma tumor cell lysate vaccine: Melacine. Semin. Cancer Biol. 2003, 13, 409–415. [Google Scholar] [CrossRef]
- Ivins, B.E.; Pitt, M.L.M.; Fellows, P.F.; Farchaus, J.W.; Benner, G.E.; Waag, D.M.; Little, S.F.; Anderson, G.W., Jr.; Gibbs, P.H.; Friedlander, A.M. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 1998, 16, 1141–1148. [Google Scholar]
- Gomeza, J.A.; Criadoa, M.T.; Ferreirosa, C.M. Bactericidal activity of antibodies elicited against the Neisseria meningitidis 37-kDA ferric binding protein (FbpA) with different adjuvants. FEMS Immunol. Med. Microbiol. 1998, 20, 79–86. [Google Scholar] [CrossRef]
- Sjolander, S.; Hansen, J.E.S.; Lovgren-Bengtsson, K.; Akerblom, L.; Morein, B. Induction of homologous virus neutralizing antibodies in guinea-pigs immunized with two human immunodeficiency virus type 1 glycoprotein gp12-iscom preparations. A comparison with other adjuvant systems. Vaccine 1996, 14, 344–352. [Google Scholar]
- Leesman, G.D. Adjuvant composition and methods for its use. US Patent 6,630,161 B1, 2003. [Google Scholar]
- Hui, G.S.; Hashimoto, C.N. Adjuvant formulations possess differing efficacy in the potentiation of antibody and cell mediated responses to a human malaria vaccine under selective immune genes knockout environment. Int. Immunopharm. 2008, 8, 1012–1022. [Google Scholar] [CrossRef]
- Reed, S.G.; Bertholet, S.; Coler, R.N.; Friede, M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009, 30, 23–32. [Google Scholar] [CrossRef]
- Fox, C.B.; Anderson, R.C.; Dutill, T.S.; Goto, Y.; Reed, S.G.; Vedvick, T. Monitoring the effects of component structure and source and formulation stability and adjuvant activity of oil-in-water emulsions. Coll. Surf. B: Biointerfaces 2008, 65, 98–105. [Google Scholar] [CrossRef]
- Goto, Y.; Bogatzki, L.Y.; Bertholet, S.; Coler, R.N.; Reed, S.G. Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine 2007, 25, 7450–7458. [Google Scholar] [CrossRef]
- D'Hondt, E.; Hehme, N.; Hanon, E.J.; Stephenne, J. Influenza vaccine. US Patent Appl 2007/0141078 A1, 2007. [Google Scholar]
- Momin, P.M.; Garcon, N.M.J. Vaccines. US Patent 6,146,632, 2000. [Google Scholar]
- Garcon, N.; Momin, P.M. Vaccines. US Patent 6,372,227 B1, 2002. [Google Scholar]
- Cohen, J.; Druilhe, P. Immunogenic compositions comprising liver stage malarial antigens. WIPO WO/2002/038176, 2002. [Google Scholar]
- Friede, M.; Garcon, N.; Ghislaine Gerard, C.M.; Hermand, P. Vaccines. US Patent 6,544,518, 2003. [Google Scholar]
- Roestenberg, M.; Remarque, E.; de Jonge, E.; Hermsen, R.; Blythman, H.; Leroy, O.; Imoukhuede, E.; Jepsen, S.; Ofori-Anyinam, O.; Faber, B.; Kocken, C.H.; Arnold, M.; Walraven, V.; Teelen, K.; Roeffen, W.; de Mast, Q.; Ballou, W.R.; Cohen, J.; Dubois, M.C.; Ascarateil, S.; van der Ven, A.; Thomas, A.; Sauerwein, R. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02. PLoS ONE 2008, 3, e3960:1–e3960:12. [Google Scholar]
- Hayden, F.G.; Howard, W.A.; Palkonyay, L.; Kieny, M.P. Report of the 5th meeting on the evaluation of pandemic influenza prototype vaccines in clinical trials: World Health Organization, Geneva, Switzerland, 12–13 February 2009. Vaccine 2009, 27, 4079–4089. [Google Scholar] [CrossRef]
- Levie, K.; Leroux-Roels, I.; Hoppenbrouwers, K.; Kervyn, A.D.; Vandermeulen, C.; Forgus, S.; Geroux-Roels, G.; Pichon, S.; Kusters, I. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J. Infect. Dis. 2008, 198, 642–649. [Google Scholar]
- Klucker, M.F.; Haensler, J.; Probeck-Quelleec, P.; Chaux, P. Thermoreversible oil-in-water emulsion. US 2007/0191314 A1, 2007. [Google Scholar]
- Suli, J.; Benisek, Z.; Elias, D.; Svrcek, S.; Ondrejkova, A.; Ondrejka, R.; Bajova, V. Experimental squalene adjuvant: I. Preparation and testing of its effectiveness. Vaccine 2004, 22, 3464–3469. [Google Scholar]
- Benisek, Z.; Suli, J.; Elias, D.; Lenhardt, L.; Ondrejkova, A.; Ondrejka, R.; Svrcek, S.; Bajova, V. Experimental squalene advjuvant II. Harmlessness and local reactogenity. Vaccine 2004, 22, 3470–3474. [Google Scholar]
- Podolski, J.S.; Martinez, M.L. Chitosan induced immunopotentiation. US Patent 5,980,912, 1999. [Google Scholar]
- Snyder, L.L.; Woo, D.V.; Triozzi, P.L.; Stevens, V.C. Synthetic hormone/growth factor subunit vaccine with application to antifertility and cancer. In Vaccine Design: The Subunit and Adjuvant Approach; Powell, M.F., Newman, M.J., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 907–930. [Google Scholar]
- Acevedo, H.F. HCG formulation. WIPO WO/2002/085311, 2002. [Google Scholar]
- Raghuvanshi, R.S.; Katare, Y.K.; Lalwani, K.; Ali, M.M.; Singh, O.; Panda, A.K. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int. J. Pharm. 2002, 245, 109–121. [Google Scholar] [CrossRef]
- Hjorth, R.N. Adjuvants for viral vaccines. US Patent 5,718,904, 1997. [Google Scholar]
- Miles, A.P.; McClellan, H.A.; Rausch, K.M.; Zhu, D.; Whitmore, M.D.; Singh, S.; Martin, L.B.; Wu, Y.; Giersing, B.K.; Stowers, A.W.; Long, C.A.; Saul, A. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 2005, 23, 2530–2539. [Google Scholar] [CrossRef]
- Lawrence, G.W.; Saul, A.; Giddy, A.J.; Kemp, R. Phase I trail in humans of an oil-based adjuvant Seppic Montanide ISA 720. Vaccine 1997, 15, 176–178. [Google Scholar]
- Aucouturier, J.; Ascarateil, S.; Dupuis, L. The use of oil adjuvants in therapeutic vaccines. Vaccine 2006, 24, S44–S45. [Google Scholar]
- Aguado, T.; Engers, H.; Pang, T.; Pink, R. Novel adjuvants currently in clinical testing November 2-4, 1998, Fondation Merieux, Annecy, France: A meeting sponsored by the World Health Organization. Vaccine 1999, 17, 2321–2328. [Google Scholar] [CrossRef]
- Langermans, J.A.M.; Schmidt, A.; Vervenne, R.A.W.; Birkett, A.J.; Calvo-Calle, J.M.; Hensmann, M.; Thornton, G.B.; Dubovsky, F.; Weiler, H.; Nardin, E.; Thomas, A.W. Effect of adjuvant on reactogenicity and long-term immunogenicity of the malaria Vaccine ICC-1132 in macaques. Vaccine 2005, 23, 4935–4943. [Google Scholar] [CrossRef]
- Uptima. TiterMax adjuvants product info sheet. Available online: http://www.interchim.com/interchim/inter_intro_chem.htm.
- Jennings, V.M. Review of selected adjuvants used in antibody production. ILAR J. 1995, 37, 119–125. [Google Scholar]
- Sigma-Aldrich, Product Information Sheet H4397, 2006.
- Sigma-Aldrich, Product Information Sheet T2684, 2009.
- Fukasawa, L.O.; Dias, W.O.; Schenkman, R.P.; Raw, I.; Tanizaki, M.M. Adjuvant can improve protection induced by OMV vaccine against Neisseria meningitidis serogroups B/C in neonatal mice. FEMS Immunol. Med. Microbiol. 2004, 41, 205–210. [Google Scholar] [CrossRef]
- Hoskinson, R.M.; Rigby, R.D.G.; Mattner, P.E. 2-component immunoadjuvant. US Patent 5,109,026, 1992. [Google Scholar]
- Fukanoki, S.; Iwakura, T.; Iwaki, S.; Matsumoto, K.; Takeda, R.; Ikeda, K.; Shi, Z.; Mori, H. Safety and efficacy of water-in-oil-in-water emulsion vaccines containing Newcastle disease virus haemagglutinin-neuraminidase glycoprotein. Avian Pathol. 2001, 30, 506–516. [Google Scholar]
- Romera, S.A.; Hilgers, L.A.; Puntel, M.; Zamorano, P.I.; Alcon, V.L.; Dus Santos, M.J.; Blanco Viera, J.; Borca, M.V.; Sadir, A.M. Adjuvant effects of sulfolipo-cyclodextrin in a squalane-in-water and water-in-mineral oil emulsions for BHV-1 vaccines in cattle. Vaccine 2001, 19, 132–141. [Google Scholar]
- Hilgers, L.A.; Lejeune, G.; Nicolas, I.; Fochesato, M.; Boon, B. Sulfolipo-cyclodextrin in squalane-in-water as a novel and safe vaccine adjuvant. Vaccine 1999, 17, 219–228. [Google Scholar] [CrossRef]
- Hilgers, L.A.T.; Blom, A.G. Sucrose fatty acid sulphate esters as novel vaccine adjuvant. Vaccine 2006, 24S2, S2/81–S2/82. [Google Scholar]
- Hilgers, L.A.T.; Blom, A.G. Mono- and disaccharide derivatives. European Patent 1,233,969 B1, 2008. [Google Scholar]
- Safety and efficacy study of angiotensin therapeutic vaccine in subjects with mild to moderate hypertension. Available online: http://clinicaltrialsgov/show/NCT00702221.
- Hariharan, K.; Hanna, N. Development and application of PROVAX adjuvant formulation for subunit cancer vaccines. Adv. Drug. Del. Rev. 1997, 32, 187–197. [Google Scholar] [CrossRef]
- Shahiwala, A.; Amiji, M.M. Enhanced mucosal and systemic immune response with squalane oil-containing multiple emulsions upon intranasal and oral administration in mice. J. Drug Target. 2008, 16, 302–310. [Google Scholar] [CrossRef]
- Yarkoni, E.; Rapp, H.J. Influence of type of oil and surfactant concentration on the efficacy of emulsified Mycobacterium bovis BCG cell walls to induce tumor regression in guinea pigs. Infect. Immun. 1980, 28, 881–886. [Google Scholar]
- Yarkoni, E.; Rapp, H.J. Tumor regression after intralesional injection of mycobacterial components emulsified in 2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene (squalene), 2,6,10,15,19,23-hexamethyltetracosane (squalane), peanut oil, or mineral oil. Cancer Res. 1979, 39, 1518–1520. [Google Scholar]
- Hauss, D.J. Oral lipid-based formulations. Adv. Drug Del. Rev. 2007, 59, 667–676. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Hata, K.; Lee, K.B.; Azuma, I. Inhibitory effect of BCG cell-wall skeletons (BCG-CWS) emulsified in squalane on tumor growth and metastasis in mice. Arch. Pharm. Res. 2002, 25, 522–527. [Google Scholar]
- Yarkoni, E.; Lederer, E.; Rapp, H.J. Immunotherapy of experimental cancer with a mixture of synthetic muramyl dipeptide and trehalose dimycolate. Infect. Immun. 1981, 32, 273–276. [Google Scholar]
- Kim, T.W.; Kim, Y.J.; Chung, H.; Kwon, I.C.; Sung, H.C.; Jeong, S.Y. The role of non-ionic surfactants on cationic lipid mediated gene transfer. J. Control. Rel. 2002, 82, 455–465. [Google Scholar] [CrossRef]
- Wang, J.J.; Sung, K.C.; Hu, O.Y.; Yeh, C.H.; Fang, J.Y. Submicron lipid emulsion as a drug delivery system for nalbuphine and its prodrugs. J. Control. Rel. 2006, 115, 140–149. [Google Scholar] [CrossRef]
- Yarkoni, E.; Rapp, H.J. Influence of surfactant concentration on the antitumor activity of components of mycobacteria. Cancer Res. 1980, 40, 975–978. [Google Scholar]
- Byars, N.E.; Nakano, G.; Welch, M.; Lehman, D.; Allison, A.C. Improvement of hepatitis B vaccine by the use of a new adjuvant. Vaccine 1991, 9, 309–318. [Google Scholar]
- Kim, Y.J.; Kim, T.W.; Chung, H.; Kwon, I.C.; Sung, H.C.; Jeong, S.Y. The effects of serum on the stability and the transfection activity of the cationic lipid emulsion with various oils. Int. J. Pharm. 2003, 252, 241–252. [Google Scholar] [CrossRef]
- Hung, C.F.; Hwang, T.L.; Chang, C.C.; Fang, J.Y. Physicochemical characterization and gene transfection efficiency of lipid emulsions with various co-emulsifiers. Int. J. Pharm. 2005, 289, 197–208. [Google Scholar] [CrossRef]
- Kwon, S.M.; Nam, H.Y.; Nam, T.; Park, K.; Lee, S.; Kim, K.; Kwon, I.C.; Kim, J.; Kang, D.; Park, J.H.; Jeong, S.Y. In vivo time-dependent gene expression of cationic lipid-based emulsion as a stable and biocompatible non-viral gene carrier. J. Control. Rel. 2008, 128, 89–97. [Google Scholar]
- Washington, C. Evaluation of non-sink dialysis methods for the measurement of drug release from colloids: effects of drug partition. Int. J. Pharm. 1989, 56, 71–74. [Google Scholar] [CrossRef]
- Takino, T.; Konishi, K.; Takakura, Y.; Hashida, M. Long circulating emulsion carrier systems for highly lipophilic drugs. Biol. Pharm. Bull. 1994, 17, 121–125. [Google Scholar] [CrossRef]
- Huang, Z.R.; Hua, S.C.; Yang, Y.L.; Fang, J.Y. Development and evaluation of lipid nanoparticles for camptothecin delivery: a comparison of solid lipid nanoparticles, nanostructured lipid carriers, and lipid emulsion. Acta Pharmacol. Sin. 2008, 29, 1094–1102. [Google Scholar] [CrossRef]
- Shively, M.L. Pharmaceutical solutions and emulsions containing taxol. US Patent 5,407,683, 1995. [Google Scholar]
- Chan, P.; Tomlinson, B.; Lee, C.B.; Lee, Y.S. Effectiveness and safety of low-dose pravastatin and squalene, alone and in combination, in elderly patients with hypercholesterolemia. J. Clin. Pharmacol. 1996, 36, 422–427. [Google Scholar]
- Xiao, W.; Wang, L.; Davis, P.J.; Liu, H. Microemulsion of seal oil markedly enhances the transfer of a hydrophobic radiopharmaceutical into acetylated low density lipoprotein. Lipids 1999, 34, 503–509. [Google Scholar] [CrossRef]
- Relas, H.; Gylling, H.; Miettinen, T.A. Fate of intravenously administered squalene and plant sterols in human subjects. J. Lipid Res. 2001, 42, 988–994. [Google Scholar]
- Fulco, C.E.; Liverman, C.T.; Sox, H.C. Gulf War and Health: Depleted Uranium, Pyridostigmine Bromide, Sarin, Vaccines; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Christian, M.S. Final report on the safety assessment of squalane and squalene. Int. J. Toxicol. 1982, 1, 37–56. [Google Scholar]
- Gherardi, R.K. Lessons from macrophagic myofasciitis: Towards definition of a vaccine adjuvant-related syndrome. Rev. Neurol. (Paris) 2003, 159, 162–164. [Google Scholar]
- Spanggord, R.J.; Sun, M.; Lim, P.; Ellis, W.Y. Enhancement of an analytical method for the determination of squalene in anthrax vaccine adsorbed formulations. J. Pharm. Biomed. Anal. 2006, 42, 494–499. [Google Scholar] [CrossRef]
- Asa, P.B.; Cao, Y.; Garry, R.F. Antibodies to squalene in Gulf War Syndrome. Exp. Mol. Pathol. 2000, 68, 55–64. [Google Scholar] [CrossRef]
- Asa, P.B.; Wilson, R.B.; Garry, R.F. Antibodies to squalene in recipients of anthrax vaccine. Exp. Mol. Pathol. 2002, 73, 19–27. [Google Scholar] [CrossRef]
- Alving, C.R.; Grabenstein, J.D. Letter to the Editor. Exp. Mol. Pathol. 2000, 68, 196–197. [Google Scholar] [CrossRef]
- Asa, P.B.; Cao, Y.; Garry, R.F. Reply. Exp. Mol. Pathol. 2000, 68, 197–198. [Google Scholar] [CrossRef]
- del Giudice, G.; Fragapane, E.; Bugarini, R.; Hora, M.; Henriksson, T.; Palla, E.; O’Hagan, D.; Donnelly, J.; Rappuoli, R.; Podda, A. Vaccines with the MF59 adjuvant do not stimulate antibody responses against squalene. Clin. Vaccine Immunol. 2006, 13, 1010–1013. [Google Scholar] [CrossRef]
- Matyas, G.R.; Wassef, N.M.; Rao, M.; Alving, C.R. Induction and detection of antibodies to squalene. J. Immunol. Methods 2000, 245, 1–14. [Google Scholar] [CrossRef]
- Matyas, G.R.; Rao, M.; Alving, C.R. Induction and detection of antibodies to squalene: II. Optimization of the assay for murine antibodies. J. Immunol. Methods 2002, 267, 119–129. [Google Scholar] [CrossRef]
- Matyas, G.R.; Rao, M.; Pittman, P.R.; Burge, R.; Robbins, I.E.; Wassef, N.M.; Thivierge, B.; Alving, C.R. Detection of antibodies to squalene: III. Naturally occurring antibodies to squalene in humans and mice. J. Immunol. Methods 2004, 286, 47–67. [Google Scholar] [CrossRef]
- Kuroda, Y.; Nacionales, D.C.; Akaogi, J.; Reeves, W.H.; Satoh, M. Autoimmunity induced by adjuvant hydrocarbon oil components of vaccine. Biomed. Pharmacother. 2004, 58, 325–337. [Google Scholar] [CrossRef]
- Phillips, C.J.; Matyas, G.R.; Hansen, C.J.; Alving, C.R.; Smith, T.C.; Ryan, M.A.K. Antibodies to squalene in US Navy Persian Gulf War veterans with chronic multisymptom illness. Vaccine 2009, 27, 3921–3926. [Google Scholar]
- Satoh, M.; Kuroda, Y.; Yoshida, H.; Behney, K.M.; Mizutani, A.; Akaogi, J.; Nacionales, D.C.; Lorenson, T.D.; Rosenbauer, R.J.; Reeves, W.H. Induction of lupus autoantibodies by adjuvants. J. Autoimmun. 2003, 21, 1–9. [Google Scholar] [CrossRef]
- Lorentzen, J.C. Identification of arthritogenic adjuvants of self and foreign origin. Scand. J. Immunol. 1999, 49, 45–50. [Google Scholar] [CrossRef]
- Gajkowska, B.; Smialek, M.; Ostrowski, R.P.; Piotrowski, P.; Frontczak-Baniewicz, M. The experimental squalene encephaloneuropathy in the rat. Exp. Toxicol. Pathol. 1999, 51, 75–80. [Google Scholar] [CrossRef]
- Asnis, D.S.; Saltzman, H.P.; Melchert, A. Shark oil pneumonia. An overlooked entity. Chest 1993, 103, 976–977. [Google Scholar]
- Klein, N. Quality control methods for oil-in-water emulsions containing squalene. WIPO WO/2008/056263 A2, 2008. [Google Scholar]
- Nenadis, N.; Tsimidou, M. Determination of squalene in olive oil using fractional crystallization for sample preparation. J. Amer. Oil Chem. Soc. 2002, 79, 257–259. [Google Scholar] [CrossRef]
- Hazotte, A.; Libong, D.; Matoga, M.; Chaminade, P. Comparison of universal detectors for high-temperature micro liquid chromatography. J. Chromatogr. A 2007, 1170, 52–61. [Google Scholar] [CrossRef]
- Masukawa, Y.; Tsujimura, H.; Imokawa, G. A systematic method for the sensitive and specific determination of hair lipids in combination with chromatography. J. Chromatogr. B 2005, 823, 131–142. [Google Scholar] [CrossRef]
- Iwata, M.; Ohno, S.; Kawai, T.; Ichijima, H.; Cavanagh, H.D. In vitro evaluation of lipids adsorbed on silicone hydrogel contact lenses using a new gas chromatography/mass spectrometry analytical method. Eye Contact Lens 2008, 34, 272–280. [Google Scholar] [CrossRef]
- Rocha, S.M.; Goncalves, V.; Evtuguin, D.; Delgadillo, I. Distinction and identification of lignins based on their volatile headspace composition. Talanta 2008, 75, 594–597. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutri. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Coll. Inter. Sci. 2004, 108-109, 227–258. [Google Scholar] [CrossRef]
- Driscoll, D.F. Lipid injectable emulsions: pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959–1969. [Google Scholar] [CrossRef]
- Norden, T.P.; Siekmann, B.; Lundquist, S.; Malmsten, M. Physicochemical characterisation of a drug-containing phospholipid-stabilised o/w emulsion for intravenous administration. Eur. J. Pharm. Sci. 2001, 13, 393–401. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fox, C.B. Squalene Emulsions for Parenteral Vaccine and Drug Delivery. Molecules 2009, 14, 3286-3312. https://doi.org/10.3390/molecules14093286
Fox CB. Squalene Emulsions for Parenteral Vaccine and Drug Delivery. Molecules. 2009; 14(9):3286-3312. https://doi.org/10.3390/molecules14093286
Chicago/Turabian StyleFox, Christopher B. 2009. "Squalene Emulsions for Parenteral Vaccine and Drug Delivery" Molecules 14, no. 9: 3286-3312. https://doi.org/10.3390/molecules14093286
APA StyleFox, C. B. (2009). Squalene Emulsions for Parenteral Vaccine and Drug Delivery. Molecules, 14(9), 3286-3312. https://doi.org/10.3390/molecules14093286