An Unexpected Reaction between 5-Hydroxymethylfurfural and Imidazolium-Based Ionic Liquids at High Temperatures
Abstract
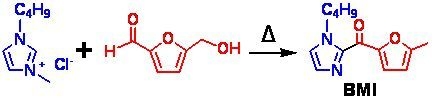
:1. Introduction
2. Results and Discussion
2.1. Structural Analysis Based on One-Dimensional NMR Spectra




2.2. Further Structural Information of the Compound BMI





2.3. Possible Mechanism for the Formation of BMI

2.4. Formation of BMI under Other Conditions
3. Experimental Section
3.1. General
3.2. Typical Procedure for Formation of the New Product
3.3. Characterization
4. Conclusions
Acknowledgements
References
- Abedin, S.Z.E.; Endres, F. Ionic liquids: The link to high-temperature molten salts? Acc. Chem. Res. 2007, 40, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Voth, G.A. Ionic liquids. Acc. Chem. Res. 2007, 40, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Morrissey, S.; Gathergood, N.; Delort, A.M.; Husson, P.; Gomes, M.F.C. The presence of functional groups key for biodegradation in ionic liquids: Effect on gas solubility. ChemSusChem 2010, 3, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Fremantle, M. Designer solvents–Ionic liquids may boost clean technology development. Chem. Eng. News 1998, 76, 32–37. [Google Scholar] [CrossRef]
- Gordon, C.M.; Holbrey, J.D.; Kennedy, A.R.; Seddon, K.R. Ionic liquid crystals: Hexafluorophosphate salts. J. Mater. Chem. 1998, 8, 2627–2636. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2285. [Google Scholar] [CrossRef]
- Wilkes, J.S. Properties of ionic liquid solvents for catalysis. J. Mol. Catal. A 2004, 214, 11–17. [Google Scholar] [CrossRef]
- Miao, W.; Chan, T.H. Ionic-liquid-supported synthesis: A novel liquid-phase strategy for organic synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.G.; Rogers, R.D. Room temperature ionic liquids as novel media for ‘clean’ liquid-liquid extraction. Chem. Commun. 1998, 16, 1765–1766. [Google Scholar] [CrossRef]
- Henderson, L.C.; Byrne, N. Rapid and efficient protic ionic liquid-mediated pinacol rearrangements under microwave irradiation. Green Chem. 2011, 13, 813–816. [Google Scholar] [CrossRef]
- Rantwijk, F.V.; Sheldon, R.A. Biocatalysis in ionic liquids. Chem. Rev. 2007, 107, 2757–2785. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquid. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of carbohydrates in ionic liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Fradea, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- James, O.O.; Maity, S.; Usman, L.A.; Ajanaku, K.O.; Ajani, O.O.; Siyanbola, T.O.; Sahu, S.; Chaubey, R. Towards the conversion of carbohydrate biomass feedstocks to biofuels via hydroxylmethylfurfural. Energy Environ. Sci. 2010, 3, 1833–1850. [Google Scholar] [CrossRef]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethylfurfural and its derivatives. ARKIVOC 2001, i, 17–54. [Google Scholar]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Zhao, H.B.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal chlorides in ionic liquid solvents convert sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Zhao, Z.K. Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquid. Bioresour. Technol. 2010, 101, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Wang, Q.; Xie, H.B.; Liu, W.J.; Zhao, Z.K. Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural by Germanium(IV) chloride in ionic liquids. ChemSusChem 2011, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Lee, Y.-Y.; Peng, W.-H.; Wu, K.C.-W. Cellulosic conversion in ionic liquids (ILs): Effects of H2O/cellulose molar ratios, temperatures, times, and different ILs on the production of monosaccharides and 5-hydroxymethylfurfural (HMF). Catal. Today 2011, 174, 65–69. [Google Scholar] [CrossRef]
- Tian, Y.K.; Deng, J.; Pan, T.; Guo, Q.X.; Fu, Y. Dehydration of glucose and fructose into 5-Hydroxymethylfurfural catalyzed by lewis acid in ionic liquids. Chin. J. Catal. 2011, 32, 997–1002. [Google Scholar]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural-a promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Shanks, B.H. Unleashing biocatalysis/chemical catalysis synergies for efficient biomass conversion. ACS Chem. Biol. 2007, 2, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.H.; Watanabe, M.; Aida, T.M.; Smith, R.L. Efficient one-pot production of 5-hydroxymethylfurfural from inulin in ionic liquids. Green Chem. 2010, 12, 1855–1860. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Song, J.; Zhou, Y.; Han, B.X. Efficient conversion of glucose into 5-hydroxymethylfurfural catalyzed by a common Lewis acid SnCl4 in an ionic liquid. Green Chem. 2009, 11, 1746–1749. [Google Scholar] [CrossRef]
- Handy, S.T. Room temperature ionic liquids: Different classes and physical properties. Curr. Org. Chem. 2005, 9, 959–988. [Google Scholar] [CrossRef]
- Canal, J.P.; Ramnial, T.; Dickie, D.A.; Clyburne, J.A.C. From the reactivity of N-heterocyclic carbenes to new chemistry in ionic liquids. Chem. Commun. 2006, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.K.; Emme, I.; Mereu, A. Unexpected side reactions of imidazolium-based ionic liquids in the base-catalysed Baylis-Hillman reactions. Chem. Commun. 2002, 1612–1613. [Google Scholar] [CrossRef]
- Ebner, G.; Schiehser, S.; Potthast, A.; Rosenau, T. Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett. 2008, 49, 7322–7324. [Google Scholar] [CrossRef]
- Li, C.Z.; Zhang, Z.H.; Zhao, Z.K. Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 2009, 50, 5403–5405. [Google Scholar] [CrossRef]
- Chan, B.K.M.; Chang, N.H.; Grimmett, M.R. The synthesis and thermolysis of imidazole quaternary salts. Aust. J. Chem. 1977, 30, 2005–2013. [Google Scholar] [CrossRef]
- Ungnade, H.E.; Crandall, E.W. The reaction of aromatic aldehydes with aluminum chloride and benzene. J. Am. Chem. Soc. 1949, 71, 2209–2210. [Google Scholar] [CrossRef]
- Yadav, I.S.; Reddy, B.V.S.; Premalatha, K. 1-Butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4) ionic liquid: A novel and recyclable reaction medium for the synthesis of vic-diamines. Adv. Synth. Catal. 2003, 345, 948–952. [Google Scholar] [CrossRef]
- Nara, S.J.; Harjani, J.R.; Salunkhe, M.M.; Mane, A.T.; Wadgaonkar, P.P. Lipase-catalysed polyester synthesis in 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. Tetrahedron Lett. 2003, 44, 1371–1373. [Google Scholar] [CrossRef]
- Lecocq, V.; Graille, A.; Santini, C.C.; Baudouin, A.; Chauvin, Y.; Basset, J.M.; Arzel, L.; Bouchu, D.; Fenet, B. Synthesis and characterization of ionic liquids based upon 1-butyl-2,3-dimethylimidazolium chloride/ZnCl2. New J. Chem. 2005, 29, 700–706. [Google Scholar] [CrossRef]
Sample Availability: Samples of the new compound are available from the authors. |
| Entry | ILs | Temperature (°C) | Product mass (mg) | HMF recovery (%) |
|---|---|---|---|---|
| 1 | [Bmim]Cl | 250 | 20 | 0 |
| 2 | [Bmim]BF4 | 250 | 0 | 0 |
| 3 | [Bmim]PF6 | 250 | 0 | 0 |
| 4 | [Bmim]NTf2 | 250 | 0 | 0 |
| 5 | [Emim]OAc | 250 | 0 | 0 |
| 6 | [Bmmim]Cl | 250 | 0 | 0 |
| 7 | [Bmim]Cl | 200 | 28 | 0 |
| 8 | [Bmim]Cl | 180 | 0 | 0 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Z.; Liu, W.; Xie, H.; Zhao, Z.K. An Unexpected Reaction between 5-Hydroxymethylfurfural and Imidazolium-Based Ionic Liquids at High Temperatures. Molecules 2011, 16, 8463-8474. https://doi.org/10.3390/molecules16108463
Zhang Z, Liu W, Xie H, Zhao ZK. An Unexpected Reaction between 5-Hydroxymethylfurfural and Imidazolium-Based Ionic Liquids at High Temperatures. Molecules. 2011; 16(10):8463-8474. https://doi.org/10.3390/molecules16108463
Chicago/Turabian StyleZhang, Zehui, Wujun Liu, Haibo Xie, and Zongbao K. Zhao. 2011. "An Unexpected Reaction between 5-Hydroxymethylfurfural and Imidazolium-Based Ionic Liquids at High Temperatures" Molecules 16, no. 10: 8463-8474. https://doi.org/10.3390/molecules16108463
APA StyleZhang, Z., Liu, W., Xie, H., & Zhao, Z. K. (2011). An Unexpected Reaction between 5-Hydroxymethylfurfural and Imidazolium-Based Ionic Liquids at High Temperatures. Molecules, 16(10), 8463-8474. https://doi.org/10.3390/molecules16108463