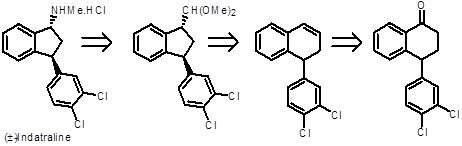
Indatraline: Synthesis and Effect on the Motor Activity of Wistar Rats
Abstract
:1. Introduction


2. Results and Discussion
2.1. Diastereoselective Synthesis of Indatraline




| Entry | HTIB | MeOH | Temperature | Time | Products (Yield) |
|---|---|---|---|---|---|
| 1 | 0.9 equiv. | anyd | r.t. | 24 h | 7 (17%); 4 (25%); 8 (26%). |
| 2 | 1.4 equiv. | anyd | r.t. | 3.5 h | 7 (35%); 8 (39%). |
| 3 | 2.8 equiv. | anyd | r.t. | 1 h | 4 (50%); 8 (39%). |
| 4 | 3.6 equiv. | anyd | r.t. | 15 min | 4 (62%); 8 35%. |
| 5 | 1.2 equiv. | 95% | r.t. | 30 min | 4 (16%); 8 (9%) |
| 6 | 2.0 equiv. | 95% | r.t. | 30 min | 4 (26%); 8 (35%) |
| 7 | 1.1 equiv. TTN | 95% | r.t. | 2 min | 4 (80%) |
| 8 | 1.1 equiv. TTN | TMOF | 0 °C | 5 min | 4 (88%) |







2.1. Motor Activity of Indatraline (1) and Indanamide (3)
2.1.1. Experiment 1


2.1.2. Experiment 2


2.1.3. Discussion
3. Experimental
3.1. Synthesis of Indatraline (1)
3.1.1. General Information
3.1.2. Deprotection of Aldehyde 10 and Its Reaction with 2-Iodobenzoic acid and Oxone
3.1.3. 1,3-trans-3-(3,4-Dichlorophenyl)-2,3-di-hydro-1H-indene-1-carbaldehyde (10)
3.1.4. 1,3-trans-3-(3,4-Dichlorophenyl)-2,3-dihydro-1H-indene-1-carbonitrile (15)
3.2. Materials and Methods
3.2.1. Animals
3.2.2. Drugs
3.2.3. Apparatus
3.2.4. Procedures
| Experiment | Drug | Group/Dose |
|---|---|---|
| 1 | Indatraline | mg/kg (8) |
| 0.5 mg/kg (8) | ||
| 1.0 mg/kg (8) | ||
| 2.0 mg/kg (8) | ||
| 3.0 mg/kg (8) | ||
| 2 | Indanamide | 0.0 mg/kg (8) |
| 0.5 mg/kg (8) | ||
| 1.0 mg/kg (8) | ||
| 2.0 mg/kg (8) | ||
| 3.0 mg/kg (8) |
3.2.5. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Hyttel, J.; Larsen, J.J. Neurochemical profile of Lu-19-005, a potent inhibitor of uptake of dopamine, noradrenaline, and serotonin. J. Neurochem. 1985, 44, 1615–1622. [Google Scholar] [CrossRef]
- Rosenzweig-Lipson, S.; Bergman, J.; Spealman, R.D.; Madras, B.K. Stereoselective behavioral-effects of Lu 19-005 in monkeys-Relation to binding at cocaine recognition sites. Psychopharmacology 1992, 107, 186–194. [Google Scholar] [CrossRef]
- Kuhar, M.J.; Ritz, M.C.; Boja, J.W. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991, 14, 299–302. [Google Scholar] [CrossRef]
- de Lima, M.S.; de Oliveira Soares, B.G.; Reisser, A.A.; Farrell, M. Pharmacological treatment of cocaine dependence: A systematic review. Addiction 2002, 97, 931–949. [Google Scholar] [CrossRef]
- Negus, S.S.; Brandt, M.R.; Mello, N.K. Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys. J. Pharmacol. Exp. Ther. 1999, 291, 60–69. [Google Scholar]
- Li, S.M.; Campbell, B.L.; Katz, J.L. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J. Pharmacol. Exp. Ther. 2006, 317, 1088–1096. [Google Scholar] [CrossRef]
- Schenk, S. Effects of GBR 12909, WIN 35,428 and indatraline on cocaine self-administration and cocaine seeking in rats. Psychopharmacology 2002, 160, 263–270. [Google Scholar] [CrossRef]
- Kleven, M.S.; Kamenka, J.M.; Vignon, J.; Koek, W. Pharmacological characterization of the discriminative stimulus properties of the phencyclidine analog, N-[1-(2-benzo(b)thiophenyl)-cyclohexyl]piperidine. Psychopharmacology 1999, 145, 370–377. [Google Scholar] [CrossRef]
- Tirelli, E.; Witkin, J.M. Differential-effects of direct and indirect dopamine agonists on the induction of gnawing in C57bl/6j mice. J. Pharmacol. Exp. Ther. 1995, 273, 7–15. [Google Scholar]
- Wise, R.A.; Bozarth, M.A. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987, 94, 469–492. [Google Scholar] [CrossRef]
- Vezina, P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004, 27, 827–839. [Google Scholar] [CrossRef]
- Bogeso, K.P.; Christensen, A.V.; Hyttel, J.; Liljefors, T. 3-Phenyl-1-indanamines-Potential antidepressant activity and potent inhibition of dopamine, norepinephrine, and serotonin uptake. J. Med. Chem. 1985, 28, 1817–1828. [Google Scholar] [CrossRef]
- Gu, X.H.; Yu, H.; Jacobson, A.E.; Rothman, R.B.; Dersch, C.M.; George, C.; Flippen-Anderson, J.L.; Rice, K.C. Design, synthesis, and monoamine transporter binding site affinities of methoxy derivatives of indatraline. J. Med. Chem. 2000, 43, 4868–4876. [Google Scholar] [CrossRef]
- Froimowitz, M.; Wu, K.-M.; Moussa, A.; Haidar, R.M.; Jurayj, J.; George, C.; Gardner, E.L. Slow-onset, long-duration 3-(3',4'-dichlorophenyl)-1-indanamine monoamine reuptake blockers as potential medications to treat cocaine abuse. J. Med. Chem. 2000, 43, 4981–4992. [Google Scholar] [CrossRef]
- Cossy, J.; Belotti, D.; Maguer, A. Synthesis of indatraline using a Suzuki cross-coupling reaction and a chemoselective hydrogenation: A versatile approach. Synlett 2003, 1515–1517. [Google Scholar]
- Pastre, J.C.; Correia, C.R.D. Remarkable electronic effect on the diastereoselectivity of the Heck reaction of methyl cinnamate with arenediazonium salts: Formal total synthesis of (+/−)-indatraline and (±)-Sertraline. Adv. Synth. Cat. 2009, 351, 1217–1223. [Google Scholar] [CrossRef]
- Taylor, J.G.; Correia, C.R.D. Stereoselective synthesis of unsymmetrical β,β-diarylacrylates by a Heck-Matsuda reaction: Versatile building blocks for asymmetric synthesis of β,β-diphenylpropanoates, 3-aryl-indole, and 4-aryl-3,4-dihydro-quinolin-2-one and formal synthesis of (−)-Indatraline. J. Org. Chem. 2011, 76, 857–869. [Google Scholar] [CrossRef]
- Norager, N.G.; Lorentz-Petersen, L.L.R.; Lyngso, L.O.; Kehler, J.; Juhl, K. Synthesis of optically pure 1-amino-3-aryl indanes exemplified by (+)-Indatraline. Synlett 2011, 1753–1755. [Google Scholar]
- Davies, H.M.L.; Gregg, T.M. Asymmetric synthesis of (+)-indatraline using rhodium-catalyzed C-H activation. Tetrahedron Lett. 2002, 43, 4951–4953. [Google Scholar] [CrossRef]
- Silva, L.F., Jr.; Siqueira, F.A.; Pedrozo, E.C.; Vieira, F.Y.M.; Doriguetto, A.C. Iodine(III)-promoted ring contraction of 1,2-dihydronaphthalenes: A diastereoselective total synthesis of (±)-indatraline. Org. Lett. 2007, 9, 1433–1436. [Google Scholar]
- Wirth, T. Hypervalent iodine chemistry-Modern developments in organic synthesis-Introduction and general aspects. In Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis; Springer-Verlag: Berlin, Germany, 2003; Volume 224, pp. 1–4. [Google Scholar]
- Silva, L.F., Jr. Hypervalent iodine-mediated ring contraction reactions. Molecules 2006, 11, 421–434. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Stang, P.J. Chemistry of Polyvalent Iodine. Chem. Rev. 2008, 108, 5299–5358. [Google Scholar] [CrossRef]
- Silva, L.F., Jr.; Olofsson, B. Hypervalent iodine reagents in the total synthesis of natural products. Nat. Prod. Rep. 2011, 28, 1722–1754. [Google Scholar] [CrossRef]
- Varvoglis, A. Hypervalent Iodine in Organic Synthesis; Academic Press ,Inc: San Diego, CA, USA, 1997. [Google Scholar]
- Koser, G.F. Hydroxy(tosyloxy)iodo]benzene and closely related iodanes: The second stage of development. Aldrichim. Acta 2000, 34, 89. [Google Scholar]
- Hawkins, J.M.; Watson, T.J.N. Asymmetric Catalysis in the Pharmaceutical Industry. Angew. Chem. Int. Ed. 2004, 43, 3224–3228. [Google Scholar] [CrossRef]
- Repinskaya, I.B.; Koltunov, K.Y.; Shakirov, M.M.; Shchegoleva, L.N.; Kopyug, V.A. Reaction of phenols and their derivatives with aromatic compounds in the presence of acidic agents. X: Rotational isomerism in the series of 2-methoxy-1-naphthalenonium ions. Russ. J. Org. Chem. 1993, 29, 803. [Google Scholar]
- Quallich, G.J.; Williams, M.T.; Friedmann, R.C. Friedel-Crafts synthesis of 4-(3, 4-dichlorophenyl)-3, 4-dihydro-1 (2H)-naphthalenone, a key intermediate in the preparation of the antidepressant sertraline. J. Org. Chem. 1990, 55, 4971. [Google Scholar] [CrossRef]
- Silva, L.F., Jr.; Sousa, R.M.F.; Ferraz, H.M.C.; Aguilar, A.M. Thallium Trinitrate-mediated ring contraction of 1,2-dihydronaphthalenes: The effect of electron-donating and electron-withdrawing groups. J. Braz. Chem. Soc. 2005, 16, 1160–1173. [Google Scholar]
- Silva, L.F., Jr.; Craveiro, M.V. A Diastereoselective total synthesis of trans-Trikentrin A: A ring contraction approach. Org. Lett. 2008, 10, 5417–5420. [Google Scholar] [CrossRef]
- Bianco, G.G.; Ferraz, H.M.C.; Costa, A.M.; Costa-Lotufo, L.V.; Pessoa, C.; de Moraes, M.O.; Schrems, M.G.; Pfaltz, A.; Silva, L.F., Jr. (+)- and (−)-Mutisianthol: First total synthesis, absolute configuration, and antitumor activity. J. Org. Chem. 2009, 74, 2561–2566. [Google Scholar]
- Carneiro, V.M.T.; Ferraz, H.M.C.; Vieira, T.O.; Ishikawa, E.E.; Silva, L.F., Jr. A ring contraction strategy toward a diastereoselective total synthesis of (+)-Bakkenolide A. J. Org. Chem. 2010, 75, 2877–2882. [Google Scholar] [CrossRef]
- Siqueira, F.A.; Ishikawa, E.E.; Fogaça, A.; Faccio, A.T.; Carneiro, V.M.T.; Soares, R.R.S.; Utaka, A.; Tébeká, I.R.M.; Bielawski, M.; Olofsson, B.; Silva, L.F., Jr. Metal-free synthesis of indanes by Iodine(III)-mediated ring contraction of 1,2-dihydronaphthalenes. J. Braz. Chem. Soc. 2011, 22, 1795–1807. [Google Scholar]
- Ferraz, H.M.C.; Silva, L.F., Jr. Reações de Contração de Anel Promovidas por Sais de Tálio(III). Quim. Nova 2000, 23, 216–224. [Google Scholar] [CrossRef]
- Ferraz, H.M.C.; Silva, L.F., Jr.; Vieira, T.O. Thallium Trinitrate-mediated ring contraction of 1,2-dihydronaphthalenes: An approach to the synthesis of Indans. Tetrahedron 2001, 57, 1709–1713. [Google Scholar]
- Ferraz, H.M.C.; Aguilar, A.M.; Silva, L.F., Jr. Model studies toward the synthesis of natural indans utilizing Thallium(III)-mediated Ring contraction reaction. Synthesis 2003, 7, 1031–1034. [Google Scholar]
- Ferraz, H.M.C.; Aguilar, A.M.; Silva, L.F., Jr. A diastereoselective total synthesis of the sesquiterpene (±)-Mutisiantol. Tetrahedron 2003, 59, 5817–5821. [Google Scholar]
- Ferraz, H.M.C.; Aguilar, A.M.; Silva, L.F., Jr.; Craveiro, M.V. Síntese de Indanos: Uma Seleção de Métodos Gerais e Eficientes. Quim. Nova 2005, 28, 703–712. [Google Scholar] [CrossRef]
- Silva, L.F., Jr.; Carneiro, V.M.T. Thallium(III) in organic synthesis. Synthesis 2010, 1059–1074. [Google Scholar]
- Silva, L.F., Jr.; Quintiliano, S.A.P.; Craveiro, M.V.; Vieira, F.Y.M.; Ferraz, H.M.C. Rearrangement of homoallylic alcohols with thallium(III): Diastereoselective synthesis of Indans bearing a beta-hydroxy ketone moiety. Synthesis 2007, 355–362. [Google Scholar]
- Satam, V.; Harad, A.; Rajule, R.; Pati, H. 2-Iodoxybenzoic acid (IBX): An efficient hypervalent iodine reagent. Tetrahedron 2010, 66, 7659–7706. [Google Scholar] [CrossRef]
- Duschek, A.; Kirsch, S.F. 2-Iodoxybenzoic acid-A simple oxidant with a dazzling array of potential applications. Angew. Chem. Int. Ed. 2011, 50, 1524–1552. [Google Scholar] [CrossRef]
- Thottumkara, A.P.; Vinod, T.K. Synthesis and oxidation reactions of a user- and eco-friendly hypervalent iodine reagent. Tetrahedron Lett. 2002, 43, 569–572. [Google Scholar] [CrossRef]
- Thottumkara, A.P.; Bowsher, M.S.; Vinod, T.K. In situ generation of o-iodoxybenzoic acid (IBX) and the catalytic use of it in oxidation reactions in the presence of oxone as a co-oxidant. Org. Lett. 2005, 7, 2933–2936. [Google Scholar] [CrossRef]
- Schulze, A.; Giannis, A. Oxidation of alcohols with catalytic amounts of IBX. Synthesis 2006, 257–260. [Google Scholar]
- Moorthy, J.N.; Singhal, N.; Senapati, K. Oxidative cleavage of vicinal diols: IBX can do what Dess-Martin periodinane (DMP) can. Org. Biomol. Chem. 2007, 5, 767–771. [Google Scholar] [CrossRef]
- Doriguetto, A.C.; Correa, R.S.; Siqueira, F.A.; Silva, L.F.; Ellena, J. Molecular conformation of the racemic indan derivative (±)-1-trans-3-(3,4-dichlorophenyl)-2,3-dihydro-1H-indene-1-carboxamide. Struct. Chem. 2009, 20, 795–800. [Google Scholar] [CrossRef]
- Shie, J.-J.; Fang, J.-M. Direct conversion of aldehydes to amides, tetrazoles, and triazines in aqueous media by one-pot tandem reactions. J. Org. Chem. 2003, 68, 1158–1160. [Google Scholar] [CrossRef]
- Loudon, G.M.; Radhakrishna, A.S.; Almond, M.R.; Blodgett, J.K.; Boutin, R.H. Conversion of aliphatic amides into amines with [I, I-bis (trifluoroacetoxy) iodo] benzene. 1. Scope of the reaction. J. Org. Chem. 1984, 49, 4272–4276. [Google Scholar] [CrossRef]
- Jacquemard, U.; Beneteau, V.; Lefoix, M.; Routier, S.; Merour, J.Y.; Coudert, G. Mild and selective deprotection of carbamates with BU4NF. Tetrahedron 2004, 60, 10039–10047. [Google Scholar] [CrossRef]
- Snider, B.B.; Lin, H. Total synthesis of (-)-FR901483. J. Am. Chem. Soc. 1999, 121, 7778. [Google Scholar] [CrossRef]
- Grieco, P.A.; Hon, Y.S.; Perez-Mendrano, A. Convergent, enantiospecific total synthesis of the novel cyclodepsipeptide (+)-jasplakinolide (jaspamide). J. Am. Chem. Soc. 1988, 110, 1630. [Google Scholar] [CrossRef]
- Nudelman, A.; Bechor, Y.; Falb, E.; Fischer, B.; Wexler, B.A.; Nudelman, A. Acetyl chloride-methanol as a convenient reagent for: a) quantitative formation of amine hydrochlorides b) carboxylate ester formation c) mild removal of N-t-Boc-protective group. Synth. Commun. 1998, 28, 471–474. [Google Scholar] [CrossRef]
- Maisonneuve, I.M.; Visker, K.E.; Mann, G.L.; Bandarage, U.K.; Kuehne, M.E.; Glick, S.D. Time-dependent interactions between iboga agents and cocaine. Eur. J. Pharmacol. 1997, 336, 123–126. [Google Scholar] [CrossRef]
- Garcia-Mijares, M.; Bernardes, A.M.T.; Silva, M.T.A. Diethylpropion produces psychostimulant and reward effects. Pharmacol. Biochem. Behav. 2009, 91, 621–628. [Google Scholar] [CrossRef]
- Farre, M.; Cami, J. Pharmacokinetic considerations in abuse liability evaluation. Br. J. Addict. 1991, 86, 1601–1606. [Google Scholar] [CrossRef]
- Javaid, J.I.; Fischman, M.W.; Schuster, C.R.; Dekirmenjian, H.; Davis, J.M. Cocaine plasma concentration: Relation to physiological and subjective effects in humans. Science 1978, 202, 227–228. [Google Scholar]
- Greenwald, M.K.; Lundahl, L.H.; Steinmiller, C.L. Sustained release d-amphetamine reduces cocaine- but not 'speedball'-seeking behavior in buprenorphine-maintained volunteers: A test of dual agonist pharmacotherapy for heroin/cocaine polydrug abusers. Neuropsychopharmacology 2010, 35, 2624–2637. [Google Scholar] [CrossRef]
- Castells, X.; Casas, M.; Pérez-Mañá, C.; Roncero, C.; Vidal, X.; Capellà, D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2010, 17, CD007380. [Google Scholar]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kameyama, M.; Siqueira, F.A.; Garcia-Mijares, M.; F. Silva, Jr., L.; Silva, M.T.A. Indatraline: Synthesis and Effect on the Motor Activity of Wistar Rats. Molecules 2011, 16, 9421-9438. https://doi.org/10.3390/molecules16119421
Kameyama M, Siqueira FA, Garcia-Mijares M, F. Silva, Jr. L, Silva MTA. Indatraline: Synthesis and Effect on the Motor Activity of Wistar Rats. Molecules. 2011; 16(11):9421-9438. https://doi.org/10.3390/molecules16119421
Chicago/Turabian StyleKameyama, Márcia, Fernanda A. Siqueira, Miriam Garcia-Mijares, Luiz F. Silva, Jr., and Maria T. A. Silva. 2011. "Indatraline: Synthesis and Effect on the Motor Activity of Wistar Rats" Molecules 16, no. 11: 9421-9438. https://doi.org/10.3390/molecules16119421
APA StyleKameyama, M., Siqueira, F. A., Garcia-Mijares, M., F. Silva, Jr., L., & Silva, M. T. A. (2011). Indatraline: Synthesis and Effect on the Motor Activity of Wistar Rats. Molecules, 16(11), 9421-9438. https://doi.org/10.3390/molecules16119421