Antioxidant Activity and Total Phenols from the Methanolic Extract of Miconia albicans (Sw.) Triana Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenols
2.2. Antioxidant Assays

| Compound | IC50 (µg/mL) ± SD |
|---|---|
| 1 | 14.94 ± 0.0040 |
| 2 | 5.93 ± 0.0035 |
| 3 | 2.97 ± 0.0105 |
| n-BuOH Fraction | 7.72 ± 0.0350 |
| MeOH Extract | 49.45 ± 0.0050 |
| Standard (rutin) | 2.55 ± 0.0075 |
| Standard (quercetin) | 1.60 ± 0.0450 |
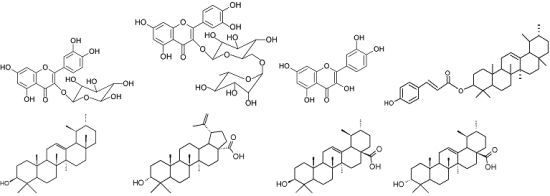
2.3. Identification of Compounds

| Carbon | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|
| 1 | 38.2 | 38.8 | 34.0 | 38.7 | 36.3 | |
| 2 | 22.8 | 27.3 | 25.1 | 27.5 | 27.5 | |
| 3 | 82.3 | 79.1 | 76.9 | 79.3 | 76.9 | |
| 4 | 39.3 | 38.8 | 38.5 | 38.7 | 38.2 | |
| 5 | 55.0 | 55.2 | 54.9 | 55.2 | 54.8 | |
| 6 | 17.5 | 18.3 | 18.0 | 18.6 | 18.1 | |
| 7 | 32.3 | 32.9 | 33.9 | 33.2 | 32.7 | |
| 8 | 40.4 | 39.6 | 41.2 | 39.1 | 38.5 | |
| 9 | 46.9 | 47.7 | 49.9 | 47.8 | 47.0 | |
| 10 | 36.9 | 36.9 | 36.7 | 36.9 | 36.5 | |
| 11 | 23.2 | 23.3 | 20.5 | 23.5 | 23.8 | |
| 12 | 125.0 | 124.4 | 27.1 | 126.1 | 124.6 | |
| 13 | 138.3 | 139.6 | 37.6 | 139.8 | 138.2 | |
| 14 | 42.0 | 42.2 | 42.6 | 42.2 | 46.9 | |
| 15 | 28.6 | 29.7 | 31.7 | 28.5 | 26.9 | |
| 16 | 26.6 | 26.6 | 36.4 | 24.4 | 26.9 | |
| 17 | 33.7 | 33.7 | 55.5 | 33.2 | 32.7 | |
| 18 | 55.9 | 59.1 | 46.7 | 52.9 | 52.4 | |
| 19 | 39.2 | 39.7 | 48.7 | 39.3 | 39.4 | |
| 20 | 39.2 | 39.6 | 150.4 | 39.3 | 39.3 | |
| 21 | 32.2 | 31.2 | 30.1 | 30.0 | 30.2 | |
| 22 | 41.5 | 41.5 | 38.3 | 23.6 | 23.8 | |
| 23 | 28.4 | 28.1 | 28.1 | 28.2 | 28.3 | |
| 24 | 16.9 | 15.6 | 15.8 | 15.7 | 15.2 | |
| 25 | 16.9 | 15.7 | 15.9 | 15.8 | 16.1 | |
| 26 | 16.8 | 16.8 | 16.0 | 17.0 | 16.9 | |
| 27 | 23.3 | 23.4 | 14.4 | 23.8 | 23.3 | |
| 28 | 28.2 | 28.1 | 177.3 | 178.3 | 178.3 | |
| 29 | 17.4 | 17.4 | 109.6 | 17.3 | 17.0 | |
| 30 | 21.0 | 21.3 | 19.0 | 21.3 | 21.1 | |
| 28OMe | 51.3 | |||||
| 1’ | 160.0 | |||||
| 3’ | 145.0 | |||||
| 4’ | 127.3 | |||||
| 5’ | 130.0 | |||||
| 6’ | 116.0 | |||||
| 7’ | 157.7 | |||||
| 8’ | 116.0 | |||||
| 9’ | 130.0 | |||||

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Determination of Total Phenolics
3.5. Determination of Antioxidant Activity
3.5.1. Erythrocyte Suspension
3.5.2. Hemolysis Assays
3.5.3. Determination of Scavenging Activity Against DPPH Radical
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Hostettmann, K.; Queiroz, E.F.; Vieira, P.C. A Importância das Plantas Medicinais: Princípios Ativos de Plantas Superiores; EdUFSCar: São Carlos, SP, Brazil, 2003; p. 152. [Google Scholar]
- Noguchi, N.; Niki, E. Forum: Therapeutic Applications of Reactive Oxygen and Nitrogen Species in Human Disease. Free Radic. Biol. Med. 2000, 28, 1538–1546. [Google Scholar] [CrossRef]
- Hussain, S.R.; Cillar, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Filho, D.W.; da Silva, E.D.; Boveris, A. Flavonoides Antioxidantes de Plantas Medicinais e Alimentos: Importância e Perspectivas Terapêuticas. In Plantas Medicinais sob a Ótica da Química Medicinal Moderna; Yunes, R.A., Calixto, J.B., Eds.; Argos: Chapecó, Brazil, 2001; pp. 317–334. [Google Scholar]
- Renner, S.S. Phylogeny and classification of the Melastomataceae and Memecylaceae. Nord. J. Bot. 1993, 13, 519–540. [Google Scholar] [CrossRef]
- Martins, A.B.; Semir, J.; Goldenberg, R.; Martins, E. O gênero Miconia Ruiz & Pav. no Estado de São Paulo. Acta Bot. Bras. 1996, 10, 267–316. [Google Scholar]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática: Guia ilustrado para identificação das famílias de Angiospermas da flora brasileira,baseado em APG II; Instituto Plantarum: Nova Odessa, São Paulo, Brazil, 2005; Volume 1, pp. 269–276. [Google Scholar]
- Vasconcelos, M.A.L.; Ferreira, M.L.D.S.; Andrade e Silva, R.; Veneziani, C.S.; Cunha, W.R. Analgesic effects of crude extracts of Miconia albicans. Boll. Chim. Farm. 2003, 142, 333–335. [Google Scholar]
- Cunha, W.R.; Crevelin, E.J.; Arantes, G.M.; Crotti, A.E.M.; Andrade E Silva, M.L.; Furtado, N.A.J.C.; Albuquerque, S.; Ferreira, D.S. A study of the tripanocidal activity of triterpene acids isolated from Miconia species. Phytother. Res. 2006, 20, 474–478. [Google Scholar] [CrossRef]
- Li, X.C.; Jacob, M.R.; Pasco, D.S.; Elsohly, H.N.; Ninrod, A.C.; Walker, L.A.; Clark, A.M. Phenolic compounds from Miconia myriantha inhibiting Candida aspartic proteases. J. Nat. Prod. 2001, 64, 1282–1285. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; Reis, M.B.; Rodrigues, J.; Santos, L.C.; Vilegas, W.; Varanda, E.A.; Dokkedal, A.L.; Cólus, I.M.S. In vivo assessment of DNA damage and protective effects of extracts from Miconia species using the comet assay and micronucleus test. Mutagenesis 2008, 23, 501–507. [Google Scholar] [CrossRef]
- Niki, E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Meth. Enzymol. 1990, 186, 100–108. [Google Scholar] [CrossRef]
- Costa, R.M.; Magalhães, A.S.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M.; Silva, B.M. Evaluation of free radical scavenging and antihemolytic activities of quince (Cydonia oblonga) leaf: A comparative study with green tea (Camellia sinensis). Food Chem. Toxicol. 2009, 47, 860–865. [Google Scholar] [CrossRef]
- Feng, J.Y.; Liu, Z.Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? J. Agric. Food Chem. 2009, 57, 11041–11046. [Google Scholar] [CrossRef]
- Ximenes, V.F.; Lopes, M.G.; Petrônio, M.S.; Regasini, L.O.; Silva, D.H.S.; Fonseca, L.M. Inhibitory effect of gallic acid and its esters on 2,2’-azobis(2-amidinopropane)hydrochloride (AAPH)-induced hemolysis and depletion of intracellular glutathione in erythrocytes. J. Agric. Food Chem. 2010, 58, 5355–5362. [Google Scholar]
- Duarte-Almeida, J.M.; Santos, R.J.; Genovese, M.I.; Lajolo, F.M. Avaliação da atividade antioxidante utilizando sistema β-caroteno/ácido linoléico e método de seqüestro de radicais DPPH. Ciênc Tecnol. Aliment. 2006, 26, 446–452. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13C NMR spectra of pentacyclic triterpenoids-A compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- Lee, V.S.Y.; Chen, C.R.; Liao, Y.W.; Tzen, J.T.C.; Chang, C.I. Structural determination and DPPH radical-scavenging activity of two acylated flavonoid tetraglycosides in oolong tea (Camellia sinensis). Chem. Pharm. Bull. 2008, 56, 851–853. [Google Scholar] [CrossRef]
- Stalikas, C.D. Phenolic Acids and Flavonoids: Occurrence and Analytical Methods. In Free Radicals and Antioxidant Protocols; Uppu, R.M., Murthy, S.N., Pryor, W.A., Parinandi, N.L., Eds.; Humana Press: New York, NY, USA, 2010; Volume 610, p. 480. [Google Scholar]
- Muschietti, L.V.; Martino, V.S. Atividades biológicas dos flavonoides naturais. In Química de produtos naturais, novos fármacos e a moderna farmacgonosia; Yunes, R.A., Filho, V.C., Eds.; Universidade Do Vale Do Itajaí: Itajaí, Brazil, 2009; pp. 189–218. [Google Scholar]
- Hidalgo, M.; Sánchez-Moreno, C.; Pascual-Teresa, S. Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Ko, F.N.; Hsiao, G.; Kuo, Y.H. Protection of oxidative hemolysis by demethyl diisoeugenol in normal and β-thalassemic red blood cells. Free Radic. Biol. Med. 1997, 22, 215–222. [Google Scholar] [CrossRef]
- Pauletti, P.M.; Castro-Gamboa, I.; Silva, D.H.S.; Young, M.C.M.; Tomazela, D.M.; Eberlin, M.N.; Bolzani, V.S. New antioxidant C-glucosylxanthone from the stems of Arrabidaea samydoides. J. Nat. Prod. 2003, 66, 1384–1387. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1-8 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pieroni, L.G.; Rezende, F.M.d.; Ximenes, V.F.; Dokkedal, A.L. Antioxidant Activity and Total Phenols from the Methanolic Extract of Miconia albicans (Sw.) Triana Leaves. Molecules 2011, 16, 9439-9450. https://doi.org/10.3390/molecules16119439
Pieroni LG, Rezende FMd, Ximenes VF, Dokkedal AL. Antioxidant Activity and Total Phenols from the Methanolic Extract of Miconia albicans (Sw.) Triana Leaves. Molecules. 2011; 16(11):9439-9450. https://doi.org/10.3390/molecules16119439
Chicago/Turabian StylePieroni, Laís Goyos, Fernanda Mendes de Rezende, Valdecir Farias Ximenes, and Anne Lígia Dokkedal. 2011. "Antioxidant Activity and Total Phenols from the Methanolic Extract of Miconia albicans (Sw.) Triana Leaves" Molecules 16, no. 11: 9439-9450. https://doi.org/10.3390/molecules16119439
APA StylePieroni, L. G., Rezende, F. M. d., Ximenes, V. F., & Dokkedal, A. L. (2011). Antioxidant Activity and Total Phenols from the Methanolic Extract of Miconia albicans (Sw.) Triana Leaves. Molecules, 16(11), 9439-9450. https://doi.org/10.3390/molecules16119439