Antimicrobial Evaluation of Diterpenes from Copaifera langsdorffii Oleoresin Against Periodontal Anaerobic Bacteria
Abstract
:1. Introduction
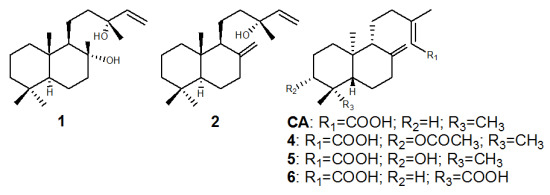
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Isolation of Compounds
3.4. Determination of the Minimal Inhibitory Concentration and Minimal Bactericidal Concentration
3.5. Time-Kill Curves
3.6. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Gursoy, U.K.; Gursoy, M.; Gursoy, O.V.; Cakmakci, L.; Kononen, E.; Uitto, V.J. Anti-biofilm properties of Satureja hortensis L. essential oil against periodontal pathogens. Anaerobe 2009, 15, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, I.; Blandino, G.; Musumeci, R.; Nicoletti, G.; Lo Bue, A.M.; Speciale, A. Antibacterial activity of moxifloxacin against periodontal anaerobic pathogens involved in systemic infections. Int. J. Antimicrob. Agents 2002, 20, 451–456. [Google Scholar] [CrossRef]
- Newman, D.J. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J. Med. Chem. 2008, 51, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, L.; Rozalski, M.; Walencka, E.; Rozalska, B.; Wysokinska, H. Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: Salvipisone as a potential anti-biofilm agent active against antibiotic resistant Staphylococci. Phytomedicine 2007, 14, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.S.B.; Murata, R.M.; Yatsuda, R.; Dos Santos, M.H.; Nagem, T.J.; Alencar, S.M.; Koo, H.; Rosalen, P.L. Antimicrobial activity of Rheedia brasiliensis and 7-epiclusianone against Streptococcus mutans. Phytomedicine 2008, 15, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Furtado, N.A.J.C.; Heleno, V.C.G.; Martins, C.H.G.; da Costa, F.B.; Severiano, M.E.; Silva, A.N.; Veneziani, R.C.S.; Ambrosio, S.R. Antimicrobial ent-pimarane diterpenes from Viguiera arenaria against Gram-positive bacteria. Fitoterapia 2009, 80, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Porto, T.S.; Rangel, R.; Furtado, N.; de Carvalho, T.C.; Martins, C.H.G.; Veneziani, R.C.S.; Da Costa, F.B.; Vinholis, A.H.C.; Cunha, W.R.; Heleno, V.C.G.; et al. Pimarane-type diterpenes: Antimicrobial activity against oral pathogens. Molecules 2009, 14, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Stavri, M.; Paton, A.; Skelton, B.W.; Gibbons, S. Antibacterial diterpenes from Plectranthus ernstii. J. Nat. Prod. 2009, 72, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. In vitro antileishmanial effects of antibacterial diterpenes from two Ethiopian Premna species: P. schimperi and P. oligotricha. BMC Pharmacol. 2003, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wiart, C.; Au, T.S.; Mohd, Y.; Hamimah, H.; Sulaiman, M. 16 Alpha hydroxy-(−)-kauran-19-oic: An antibacterial diterpene from sweet apple (Annona squamosa L., Annonaceae). Int. J. Pharmacol. 2005, 1, 296–298. [Google Scholar]
- Radulovic, N.; Denic, M.; Stojanovic-Radic, Z. Antimicrobial phenolic abietane diterpene from Lycopus europaeus L. (Lamiaceae). Bioorg. Med. Chem. Lett. 2010, 20, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, G.M.; Wächter, G.; Singh, M.P.; Maiese, W.M.; Timmermann, B.N. Antibacterial diterpenes from Calceolaria pinifolia. J. Nat. Prod. 2003, 66, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kalpoutzakis, E.; Chinou, I.; Mitaku, S.; Skaitsounis, A.L.; Hervala, C. Antibacterial labdane-type diterpenes from the resin "Ladano" of Cistus creticus Subsp. creticus. Nat. Prod. Lett. 1998, 11, 173–179. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Simao, M.R.; Ambrosio, S.R.; Furtado, N.A.; Veneziani, R.C.; Heleno, V.C.; Da Costa, F.B.; Gomes, B.P.; Souza, M.G.; Borges dos Reis, E.; et al. Antimicrobial activity of diterpenes from Viguiera arenaria against endodontic bacteria. Molecules 2011, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.B.; Martins, C.H.G.; Souza, M.G.M.; Furtado, N.A.J.C.; Heleno, V.C.G.; Sousa, J.P.B.D.; Rocha, E.M.P.; Bastos, J.K.; Cunha, W.R.; Veneziani, R.C.S.; et al. Antimicrobial activity of terpenoids from Copaifera langsdorffii Desf. against cariogenic bacteria. Phytother. Res. 2011, 25, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, A.; Yan, L.T.; Ito, S.; Edatsugi, H.; Iwata, D.; Komoda, Y. The isolation and in-vivo potent antitumor-activity of clerodane diterpenoid from the oleoresin of the Brazilian medicinal plant, Copaiferalangsdorfii Desfon. Bioorg. Med. Chem. 1994, 4, 2889–2892. [Google Scholar] [CrossRef]
- Braun, S.; Breitenbach, H. Strukturaufklärung einer neuen Diterpensäure aus Metasequoia glyptostroboides mit hilfe der 13C-NMR-spektroskopie. Tetrahedron 1977, 33, 145–150. [Google Scholar] [CrossRef]
- Romero, A.L.; Baptistella, L.H.B.; Imamura, P.M. Absolute configuration of some dinorlabdanes from copaiba oil. J. Brazil Chem. Soc. 2009, 20, 1036–1040. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F.; King, R.M. Diterpenes and norditerpenes from the aristeguetia group. Phytochemistry 1991, 30, 2991–3000. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, Approved standard; CLSI: Wayne, PA, USA, 2007. [Google Scholar]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S. Phytochemicals for bacterial resistance—Strengths, weaknesses and opportunities. Planta. Med. 2008, 74, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.; Dodds, M.; Tian, M. Naturally occurring phenolic antibacterial compounds show effectiveness against oral bacteria by a quantitative structure-activity relationship study. J. Agric. Food. Chem. 2008, 56, 11151–11156. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, A.; Rezende, M.C.; Mascayano, C.; Vasquez, L. A structure-activity study of antibacterial diterpenoids. Molecules 2008, 13, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Bae, J.; Lee, D.S. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res. 2008, 22, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Tsui, V.W.K.; Wong, R.W.K.; Rabie, A.B.M. The inhibitory effects of naringrin on the growth of periodontal pathogens in vitro. Phytother. Res. 2008, 22, 401–406. [Google Scholar] [CrossRef] [PubMed]
- More, G.; Tshikalange, T.E.; Lall, N.; Botha, F.; Meyer, J.J.M. Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol. 2008, 119, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Tsukiyama, R.I.; Suzuki, A.; Kobayashi, M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001, 45, 3009–3013. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, M.; Ginestra, G.; Mandalari, G.; Furneri, P.M.; Bisignano, G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine 2010, 17, 317–322. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Microorganism (ATCC) | Minimum inhibitory concentration in µg mL−1 [µM] | ||||||
|---|---|---|---|---|---|---|---|
| # CHD | 1 | 2 | CA | 4 | 5 | 6 | |
| B. fragilis (25285) | 7.4 [12.8] | * | 25.0 [86.1] | 25.0 [82.1] | 100.0 [275.9] | 400.0 [1248.3] | 400.0 [1196.0] |
| A. naeslundii (19039) | 1.8 [3.1] | 12.5 [40.8] | 25.0 [86.1] | 6.2 [20.4] | 25.0 [69.0] | 100.0 [312.0] | 50.0 [149.5] |
| P. gingivalis (33277) | 0.9 [1.6] | 6.2 [20.2] | 6.2 [21.3] | 3.1 [10.2] | 25.0 [69.0] | 25.0 [78.0] | 50.0 [149.5] |
| P. nigrescens (33563) | 0.9 [1.6] | 400.0 [1304.9] | 12.5 [43.0] | 200.0 [656.9] | 200.0 [551.7] | 200.0 [624.1] | 200.0 [598.0] |
| F. nucleatum (25586) | 1.8 [3.1] | 400.0 [1304.9] | 50.0 [172.1] | 200.0 [656.9] | 200.0 [551.7] | 200.0 [624.1] | 200.0 [598.0] |
| B. thetaiotaomicron (29741) | 29.0 [50.1] | * | 50.0 [172.1] | * | 400.0 [1103.4] | 400.0 [1248.2] | * |
| P. anaerobius (27337) | 7.4 [12.8] | 3.1 [10.1] | 12.5 [43.0] | 3.1 [10.2] | 12.5 [34.5] | 25.0 [78.0] | 25.0 [74.7] |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Souza, A.B.; Souza, M.G.M.d.; Moreira, M.A.; Moreira, M.R.; Furtado, N.A.J.C.; Martins, C.H.G.; Bastos, J.K.; Santos, R.A.d.; Heleno, V.C.G.; Ambrosio, S.R.; et al. Antimicrobial Evaluation of Diterpenes from Copaifera langsdorffii Oleoresin Against Periodontal Anaerobic Bacteria. Molecules 2011, 16, 9611-9619. https://doi.org/10.3390/molecules16119611
Souza AB, Souza MGMd, Moreira MA, Moreira MR, Furtado NAJC, Martins CHG, Bastos JK, Santos RAd, Heleno VCG, Ambrosio SR, et al. Antimicrobial Evaluation of Diterpenes from Copaifera langsdorffii Oleoresin Against Periodontal Anaerobic Bacteria. Molecules. 2011; 16(11):9611-9619. https://doi.org/10.3390/molecules16119611
Chicago/Turabian StyleSouza, Ariana B., Maria G. M. de Souza, Maísa A. Moreira, Monique R. Moreira, Niege A. J. C. Furtado, Carlos H. G. Martins, Jairo K. Bastos, Raquel A. dos Santos, Vladimir C. G. Heleno, Sergio Ricardo Ambrosio, and et al. 2011. "Antimicrobial Evaluation of Diterpenes from Copaifera langsdorffii Oleoresin Against Periodontal Anaerobic Bacteria" Molecules 16, no. 11: 9611-9619. https://doi.org/10.3390/molecules16119611
APA StyleSouza, A. B., Souza, M. G. M. d., Moreira, M. A., Moreira, M. R., Furtado, N. A. J. C., Martins, C. H. G., Bastos, J. K., Santos, R. A. d., Heleno, V. C. G., Ambrosio, S. R., & Veneziani, R. C. S. (2011). Antimicrobial Evaluation of Diterpenes from Copaifera langsdorffii Oleoresin Against Periodontal Anaerobic Bacteria. Molecules, 16(11), 9611-9619. https://doi.org/10.3390/molecules16119611