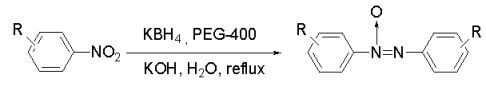
Reduction of Nitroarenes to Azoxybenzenes by Potassium Borohydride in Water
Abstract
:1. Introduction

2. Results and Discussion
| Entry | Catalyst a | Reaction time (h) | Yield (%) b |
|---|---|---|---|
| 1-1 | 0 | 23 | 52 |
| 1-2 | PEG-400 | 3 | 85 |
| 1-3 | PEG-600 | 3 | 85 |
| 1-4 | PEG-800 | 3 | 85 |
| 1-5 | PEG-1000 | 3 | 85 |
| Entry | R | Nitroarenes | m. p. (°C) b | Reaction time (h) | Products | Yield (%) c |
|---|---|---|---|---|---|---|
| 2-1 | m-Cl | 1a | 43–45 | 3 | 2a | 85 |
| 2-2 | p-Cl | 1b | 81–83 | 5 | 2b | 78 |
| 2-3 | m-Br | 1c | 56–58 | 2 | 2c | 84 |
| 2-4 | m-I | 1d | 36–38 | 3 | 2d | 80 |
| 2-5 | p-Br | 1e | 125–127 | 10 | 2e | 14 |
| 2-6 | p-Br | 1e | 125–127 | 4 | 2e | 79 d |
| 2-7 | p-I | 1f | 171–173 | 10 | 2f | 16 |
| 2-8 | p-I | 1f | 171–173 | 4 | 2f | 76 d |
| 2-9 | p-COOH | 1g | 239–241 | 2 | 2g | 92 |
| 2-10 | H | 1h | 5.7 | 4 | 2h | 61 |
| 2-11 | m-CH3 | 1i | 16 | 10 | 2i | 50 |
| 2-12 | o-CH3 | 1j | −9.5 | 24 | NR | 0 |
| 2-13 | p-CH3 | 1k | 51–52 | 24 | NR | 0 |
| 2-14 | p-OCH3 | 1l | 51–53 | 24 | NR | 0 |
3. Experimental
3.1. General
3.2. General Synthetic Procedure
4. Conclusions
Acknowledgements
References
- Weill, C.E.; Panson, G.S. The reduction of nitrobenzene to azoxybenzene by sodium borohydride. J. Org. Chem. 1956, 21, 803. [Google Scholar]
- Hutchins, R.O.; Lamson, D.W.; Rua, L.; Milewski, C.; Maryanoff, B. Reduction of aromatic nitro compounds with sodium borohydride in dimethyl sulfoxide or sulfolane. Synthesis of azo or azoxy derivatives. J. Org. Chem. 1971, 36, 803–806. [Google Scholar]
- Shine, H.J.; Mallory, H.E. The reduction of aromatic nitro compounds by potassium borohydride. J. Org. Chem. 1962, 27, 2390–2391. [Google Scholar] [CrossRef]
- Ren, P.D.; Pan, S.F.; Dong, T.W.; Wu, S.H. Reduction of aromatic nitro compounds to azoxy compounds with NaBH4/BiCl3 system. Acta Chim. Sinica 1998, 56, 714–718. [Google Scholar]
- Ren, P.D.; Pan, S.F.; Dong, T.W.; Wu, S.H. Catalytic reduction of nitroarenes to azoxybenzenes with sodium borohydride in the presence of bismuth. Synth. Commun. 1996, 26, 3903–3908. [Google Scholar] [CrossRef]
- Siemeling, U.; Türk, T.; Vorfeld, U.; Fink, H. Selective reduction of an aromatic nitro group to an azoxy Unit in the presence of an aliphatic nitro group. Monatsh. Chem. 2003, 134, 419–423. [Google Scholar] [CrossRef]
- Ohe, K.; Uemura, S.; Sugita, N.; Masuda, H.; Taga, T. Sodium arenetellurolate-catalyzed selective conversion of nitro aromatics to aromatic azoxy or azo compounds and its application for facile preparation of 3,3'- and 4,4'-bis[.beta.-(aryltelluro)vinyl] azobenzenes from (3- and 4-nitrophenyl)acetylenes. J. Org. Chem. 1989, 54, 4169–4174. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Sukumar, P.; Chandani, B. Linear unsubstituted polyethylene glycols as phase transfer catalysts. Tetrahedron Lett. 1979, 20, 3543–3544. [Google Scholar] [CrossRef]
- Zupancic, B.G.; Kokalj, M. Catalytic activity of polyethylene glycols in the reduction of carbonyl compounds under phase-transfer catalyzed conditions. Synth. Commun. 1982, 12, 881–886. [Google Scholar] [CrossRef]
- Neumann, R.; Sasson, Y. Mechanism of base-catalyzed reactions in phase-transfer systems with poly(ethy1ene glycols) as catalysts. The isomerization of allylanisole. J. Org. Chem. 1984, 49, 3448–3451, and references cited therein. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Marco, M.; Tominack, B.J. First examples of transition-metal free Sonogashira-type couplings. Org. Lett. 2003, 5, 3919–3922. [Google Scholar] [CrossRef]
- Yadav, G.D.; Motirale, B.G. Microwave-irradiated synthesis of nitrophen using PEG 400 as phase transfer catalyst and solvent. Org. Proc. Res. Devel. 2009, 13, 341–348, and references cited therein. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Z.Z. New phase transfer catalyzing reagents-polyethylene glycol and its derivatives. Huaxue Shiji 1985, 7, 20–22. [Google Scholar]
- Huang, Z.Z.; Ye, S.; Xia, W.; Yu, Y.H.; Tang, Y. Wittig-type olefination catalyzed by PEG-telluride. J. Org. Chem. 2002, 67, 3096–3103. [Google Scholar] [CrossRef]
- Mckillop, A.; Raphael, R.A.; Taylor, E.C. Thallium in organic synthesis. XI. Preparation of azoxy compounds. J. Org. Chem. 1970, 35, 1670–1672. [Google Scholar] [CrossRef]
- Hou, Z.M.; Fujiwara, Y.; Taniguchi, H. Lanthanides in organic synthesis. Samarium metal promoted selective formation of azoxy compounds. J. Org. Chem. 1988, 53, 3118–3120. [Google Scholar] [CrossRef]
- Gore, P.H.; Wheeler, O.H. The absorption spectra of aromatic azo and related compounds. I. azoxybenzenes. J. Am. Chem. Soc. 1956, 78, 2160–2163. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.; Liu, B.; Guo, A.; Dong, Z.; Jin, S.; Lu, Y. Reduction of Nitroarenes to Azoxybenzenes by Potassium Borohydride in Water. Molecules 2011, 16, 3563-3568. https://doi.org/10.3390/molecules16053563
Liu Y, Liu B, Guo A, Dong Z, Jin S, Lu Y. Reduction of Nitroarenes to Azoxybenzenes by Potassium Borohydride in Water. Molecules. 2011; 16(5):3563-3568. https://doi.org/10.3390/molecules16053563
Chicago/Turabian StyleLiu, Yufang, Bo Liu, Ailing Guo, Zhenming Dong, Shuo Jin, and Yun Lu. 2011. "Reduction of Nitroarenes to Azoxybenzenes by Potassium Borohydride in Water" Molecules 16, no. 5: 3563-3568. https://doi.org/10.3390/molecules16053563
APA StyleLiu, Y., Liu, B., Guo, A., Dong, Z., Jin, S., & Lu, Y. (2011). Reduction of Nitroarenes to Azoxybenzenes by Potassium Borohydride in Water. Molecules, 16(5), 3563-3568. https://doi.org/10.3390/molecules16053563