HPLC Separation of All Aldopentoses and Aldohexoses on an Anion-Exchange Stationary Phase Prepared from Polystyrene-Based Copolymer and Diamine: The Effect of NaOH Eluent Concentration
Abstract
:1. Introduction

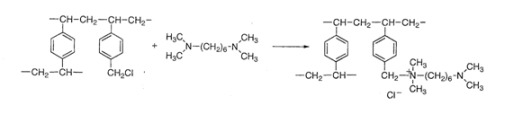
2. Results and Discussion
| Aldose | pKa [21] (25 °C) | temperature (°C) | pyranose | furanose | ||
|---|---|---|---|---|---|---|
| α (%) | β (%) | α (%) | β (%) | |||
| ribose | 12.11 | 31 | 21.5 | 58.5 | 6.5 | 13.5 |
| arabinose | 12.34 | 31 | 60 | 35.5 | 2.5 | 2 |
| xylose | 12.15 | 31 | 36.5 | 63 | <1 | <1 |
| lyxose | 12.11 | 31 | 70 | 28 | 1.5 | 0.5 |
| allose | 31 | 14 | 77.5 | 3.5 | 5 | |
| altrose | 22 | 27 | 43 | 17 | 13 | |
| glucose | 12.28 | 31 | 38 | 62 | ・・ | 0.14 |
| mannose | 12.08 | 44 | 64.9 | 34.2 | 0.6 | 0.3 |
| gulose | 22 | 16 | 81 | ・・ | 3 | |
| idose | 31 | 38.5 | 36 | 11.5 | 14 | |
| galactose | 12.35 | 31 | 30 | 64 | 2.5 | 3.5 |
| talose | □ | 22 | 42 | 29 | 16 | 13 |







3. Experimental
3.1. Materials
3.2. Equipment
3.3. Chromatographic Conditions and Measurements
4. Conclusions
Acknowledgments
References and Notes
- Collins, P.; Ferrier, R. Monosaccharides; John Wiley & Sons: Chichester, UK, 1995; Volume Chapter 2, p. 15. [Google Scholar]
- Johnson, D.C.; Lacourse, W.R. Carbohydrate Analysis; Rassi, Z.E., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; Volume Chapter 10, p. 391. [Google Scholar]
- Chaplin, M.F. Carbohydrates Analysis-A Practical Approach Series, 2nd; Chaplin, M.F., Kennedy, J.F., Eds.; Oxford University Press: Oxford, UK, 1994; Volume Chapter 1, p. 22. [Google Scholar]
- Lindhorst, T.K. Essentials of Carbohydrate Chemistry and Biochemistry; Wiley-VCH: Weinheim, Germany, 2007; Volume Chapter 2, p. 5. [Google Scholar]
- White, G.W.; Katona, T.; Zodda, J.P. The use of high-performance size exclusion chromatography (HPSEC) as a molecular weight screening technique for polygalacturonic acid for use in pharmaceutical applications. J. Pharm. Biomed. Anal. 1999, 20, 905–912. [Google Scholar] [CrossRef]
- Soga, T.; Serwe, M. Determination of carbohydrates in food samples by capillary electrophoresis with indirect UV detection. Food Chem. 2000, 69, 339–344. [Google Scholar] [CrossRef]
- Lee, Y.C. High-performance anion-exchange chromatography for carbohydrate analysis. Anal. Biochem. 1990, 189, 151–162. [Google Scholar]
- LaCourse, W.R. Pulsed electrochemical detection at noble metal electrodes in high performance liquid chromatography. Analusis 1993, 21, 181–195. [Google Scholar]
- Lee, Y.C. Carbohydrate analyses with high-performance anion-exchange chromatography. J. Chromatogr. 1996, 720, 137–149. [Google Scholar]
- Lacourse, W.R. Pulsed Electrochemical Detection; Wiley-Interscience: New York, NY, USA, 1997; Volume Chapter 7, p. 182. [Google Scholar]
- Lee, Y.C. Carbohydrate Analysis by Modern Chromatography and Electrophoresis; Rassi, Z.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume Chapter 6, p. 208. [Google Scholar]
- Cataldi, T.R.I.; Campa, C.; De Benedetto, G.E. Carbohydrate analysis by high-performance anion-exchange chromatography with pulsed amperometric detection: The potential is still growing. Fresenius J. Anal. Chem. 2000, 368, 739–758. [Google Scholar] [CrossRef]
- Weizhadler, M.; Barreto, V.; Pohl, C.; Jandik, P.; Cheng, J.; Avdaloric, N. CarboPac™ PA20: A new monosaccharide separator column with electrochemical detection with disposable gold electrodes. J. Biochem. Biophys. Methods 2004, 60, 309–317. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Shi, Y.; Liang, L.; Mou, S. Determination of several sugars in serum by high-performance anion-exchange chromatography with pulsed amperometric detection. J. Chromatogr. A 2005, 1085, 98–103. [Google Scholar]
- Caseiro, A.; Marr, I.L.; Claeys, M.; Kasper-Giebl, A.; Puxbaum, H.; Pio, C.A. Determination of saccharides in atmospheric aerosol using anion-exchange high-performance liquid chromatography and pulse-amperometric detection. J. Chromatogr. A 2007, 1171, 37–45. [Google Scholar]
- Lee, D.P.; Bunker, M.T. Carbohydrate analysis by ion chromatography. J. Chromatogr. Sci. 1989, 27, 496–503. [Google Scholar]
- Corradini, C.; Corradini, D.; Huber, C.G.; Bonn, G.K. Synthesis of a polymeric-based stationary phase for carbohydrate separation by high-pH anion-exchange chromatography with pulsed amperometric detection. J. Chromatogr.A 1994, 685, 213–220. [Google Scholar] [CrossRef]
- Masuda, T.; Nishimura, Y.; Tonegawa, M.; Kitahara, K.; Arai, S.; Yamashita, J.; Takai, N. High-performance liquid chromatographic separation of monosaccharides and disaccharides on stationary phases prepared from polystyrene-based resins and tertiary diamines. Chem. Lett. 1997, 12, 1239–1240. [Google Scholar]
- Masuda, T.; Nishimura, Y.; Tonegawa, M.; Kitahara, K.; Arai, S.; Yamashita, J.; Takai, N. High-performance liquid chromatographic separation of carbohydrates on stationary phases prepared from polystyrene-based resin and tertiary amines: Effect of chemical structure of anion-exchange sorbents. J. Chromatogr. A 1999, 845, 401–408. [Google Scholar]
- Masuda, T.; Kitahara, K.; Aikawa, Y.; Arai, S. High-performance liquid chromatographic separation of carbohydrates on a stationary phase prepared from polystyrene-based resin and novel amine. J. Chromatogr. A 2002, 961, 89–96. [Google Scholar] [CrossRef]
- Speight, J.G. Lange’s Handbook of Chemistry, 16th ed; McGraw Hill: New York, NY, USA, 2005. [Google Scholar]
- Collins, P.; Ferrier, R. Monosaccharides; John Wiley & Sons: Chichester, UK, 1995; Volume Chapter 2, p. 41. [Google Scholar]
- Mcguire, J.M.; Stewart, Y.M.; Smith, K.D. The effect of pH on the high pH anion-exchange chromatography elution of monosaccharides. Chromatographia 1999, 49, 699–702. [Google Scholar] [CrossRef]
- Rendleman, J.A. Carbohydrates in solution. Adv. Chem. Ser. 1973, 117, 51–69. [Google Scholar] [CrossRef]
- Koizumi, K.; Kubota, Y.; Ozaki, H.; Shigenobu, K.; Fukuda, M.; Tanimoto, T. Analyses of isomeric mono-O-methyl--glucoses, -glucobioses and -glucos monophosphates by high-performance anion-exchange chromatography with pulsed amperometric detection. J. Chromatogr. 1992, 595, 340–345. [Google Scholar] [CrossRef]
- Rohrer, J.S.; Olechno, J.D. Secondary isotope effect: The resolution of deuterated glucoses by anion-exchange chromatography. Anal. Chem. 1992, 64, 914–916. [Google Scholar] [CrossRef]
- Marioli, M.; Luo, P.F.; Kuwana, T. Nickel-chromium alloy electrode as a carbohydrate detector for liquid chromatography. Anal. Chim. Acta 1993, 282, 571–580. [Google Scholar] [CrossRef]
- Luo, P.F.; Kuwana, T. Nickel-titanium alloy electrode as a sensitive and stable LCEC detector for carbohydrates. Anal. Chem. 1994, 66, 2775–2782. [Google Scholar] [CrossRef]
- Luo, P.F.; Kuwana, T.; Paul, D.K.; Sherwood, P.M.A. Electrochemical and XPS study of the nickel-titanium electrode surface. Anal. Chem. 1996, 68, 3330–3337. [Google Scholar] [CrossRef]
- Samples Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Inoue, K.; Kitahara, K.-i.; Aikawa, Y.; Arai, S.; Masuda-Hanada, T. HPLC Separation of All Aldopentoses and Aldohexoses on an Anion-Exchange Stationary Phase Prepared from Polystyrene-Based Copolymer and Diamine: The Effect of NaOH Eluent Concentration. Molecules 2011, 16, 5905-5915. https://doi.org/10.3390/molecules16075905
Inoue K, Kitahara K-i, Aikawa Y, Arai S, Masuda-Hanada T. HPLC Separation of All Aldopentoses and Aldohexoses on an Anion-Exchange Stationary Phase Prepared from Polystyrene-Based Copolymer and Diamine: The Effect of NaOH Eluent Concentration. Molecules. 2011; 16(7):5905-5915. https://doi.org/10.3390/molecules16075905
Chicago/Turabian StyleInoue, Kadumi, Kei-ichi Kitahara, Yoshihiro Aikawa, Sadao Arai, and Takako Masuda-Hanada. 2011. "HPLC Separation of All Aldopentoses and Aldohexoses on an Anion-Exchange Stationary Phase Prepared from Polystyrene-Based Copolymer and Diamine: The Effect of NaOH Eluent Concentration" Molecules 16, no. 7: 5905-5915. https://doi.org/10.3390/molecules16075905
APA StyleInoue, K., Kitahara, K. -i., Aikawa, Y., Arai, S., & Masuda-Hanada, T. (2011). HPLC Separation of All Aldopentoses and Aldohexoses on an Anion-Exchange Stationary Phase Prepared from Polystyrene-Based Copolymer and Diamine: The Effect of NaOH Eluent Concentration. Molecules, 16(7), 5905-5915. https://doi.org/10.3390/molecules16075905