Synthesis, FTIR, 13C-NMR and Temperature-Dependent 1H‑NMR Characteristics of Bis-naphthalimide Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis

2.2. FTIR Spectra Study

| Compound | νasC=O | νsC=O | Others bands |
|---|---|---|---|
| [cm−1] | [cm−1] | [cm−1] | |
| NP2 | 1710 | 1681, 1667 | |
| NP3 | 1695 | 1659 | 3313 a |
| NP4 | 1702 | 1659 | |
| NP5 | 1701 | 1656 | |
| NP6 | 1698 | 1656 | 2600 b |
| NP7 | 1698 | 1659 | 2459 c |
| NP8 | 1696 | 1654 | 1741 d |
| NP9 | 1701 | 1653 |



2.3. 13C-NMR Spectra
| Compound | Chemical 13C-NMR shift [ppm] | |||
|---|---|---|---|---|
| C-4 | C-3 | C-2 | C=O | |
| NP2 * | 136.6 | 127.2 | 133.5 | 167.1 |
| NP3 | 133.6 | 126.8 | 131.1 | 164.0 |
| NP4 | 133.7 | 126.8 | 131.1 | 164.1 |
| NP5 | 133.7 | 126.8 | 131.1 | 164.1 |
| NP6 | 134.2 | 127.0 | 131.4 | 164.1 |
| NP7 | 134.2 | 126.9 | 131.5 | 164.1 |
| NP8 * | 137.7 | 128.1 | 134.1 | 167.8 |
| NP9 * | 136.7 | 127.2 | 133.1 | 166.9 |
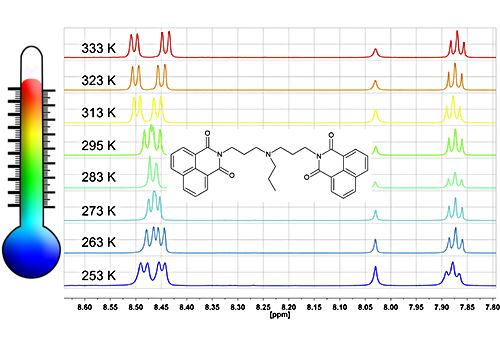
2.4. 1H-NMR Spectra












3. Experimental
3.1. General Procedures
3.2. Synthesis
4. Conclusions
Acknowledgments
References
- Cacialli, F.; Friend, R.H.; Bouche, C.M.; Le Barny, P.; Facoetti, H.; Soyer, F.; Robin, P. Naphthalimide side-chain polymers for organic light-emitting diodes: Band-offset engineering and role of polymer thickness. J. Appl. Phys. 1998, 83, 2343–2356. [Google Scholar]
- Qian, X.H.; Zhu, Z.H.; Chen, K.C. The synthesis, application and prediction of stokes shift in fluorescent dyes derived from 1,8-naphthalic anhydride. Dyes Pigm. 1989, 11, 13–20. [Google Scholar] [CrossRef]
- Martin, E.; Weigand, R.; Pardo, A. Solvent dependence of the inhibition of intramolecular charge-transfer in N-substituted 1,8-naphthalimide derivatives as dye lasers. J. Lumin. 1996, 68, 157–164. [Google Scholar] [CrossRef]
- Tao, Z.F.; Qian, X. Naphthalimide hydroperoxides as photonucleases: Substituent effects and structural basis. Dyes Pigm. 1999, 43, 139–145. [Google Scholar] [CrossRef]
- Martynski, T.; Mykowska, E.; Bauman, D. Spectral properties of fluorescent dyes in nematic liquid crystals. J. Mol. Struct. 1994, 325, 161–167. [Google Scholar] [CrossRef]
- Chang, S.C.; Archer, B.J.; Utecht, R.E.; Lewis, D.E.; Judy, M.M.; Matthews, J.L. 4-Alkylamino-3-bromo-N-alkyl-1,8-naphthalimides: New photochemically activatable antiviral compounds. Bioorg. Med. Chem. Lett. 1993, 4, 555–556. [Google Scholar]
- Peters, T.; Bide, M.J. Amino derivatives of 1,8-naphthalic anhydride and derived dyes for synthetic-polymer fibres. Dyes Pigm. 1985, 6, 349–375. [Google Scholar] [CrossRef]
- Konstantinova, T.; Meallier, P.; Grabtchev, I. The synthesis of some 1,8-naphthalic anhydride derivatives as dyes for polymeric materials. Dyes Pigm. 1993, 22, 191–198. [Google Scholar] [CrossRef]
- Gancedo, G.; Gil-Fernandez, C.; Vilas, P.; Perez, S.; Paez, E.; Rodriguez, F.; Braña, M.F.; Roldan, C.M. Imide derivatives of 3-nitro-1.8-naphthalic acid: Their inhibitory activity against DNA viruses. Arch. Virol. 1982, 74, 157–165. [Google Scholar]
- Miller, T.; Weragoda, R.; Izbicka, E.; Iverson, B.L. The synthesis and screening of 1,4,5,8-naphthalenetetracarboxylic diimide-peptide conjugates with antibacterial activity. Bioorg. Med. Chem. 2001, 9, 2015–2024. [Google Scholar] [CrossRef]
- Muth, M.; Hoerr, V.; Glaser, M.; Ponte-Sucre, A.; Moll, H.; Stich, A.; Holzgrabe, U. Antitrypanosomal activity of quaternary naphthalimide derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 1590–1593. [Google Scholar] [CrossRef]
- Braña, M.F.; Ramos, A. Naphthalimides as anti-cancer agents: Synthesis and biological activity. Curr. Med. Chem. 2001, 1, 237–255. [Google Scholar]
- Braña, M.F.; Cacho, M.; Gradillas, A.; de Pascual-Teresa, B.; Ramos, A. Intercalators as anticancer drugs. Curr. Pharm. Des. 2001, 7, 1745–1780. [Google Scholar] [CrossRef]
- Martinez, R.; Chacón-Garcia, L. The search of DNA-intercalators as antitumoral drugs: What it worked and what did not work. Curr. Med. Chem. 2005, 12, 127–151. [Google Scholar]
- Sami, S.M.; Dorr, R.T.; Alberts, D.S.; Remers, W.A. 2-Substituted 1,2-dihydro-3H-dibenz[de,h]isoquinoline-1,3-diones. A new class of antitumor agent. J. Med. Chem. 1993, 36, 765–770. [Google Scholar] [CrossRef]
- Sami, S.M.; Dorr, R.T.; Alberts, D.S.; Sólyom, A.M.; Remers, W.A. Analogues of amonafide and azonafide with novel ring systems. J. Med. Chem. 2000, 43, 3067–3073. [Google Scholar] [CrossRef]
- Braña, M.F.; Cacho, M.; Garcia, M.A. Synthesis, antitumor activity, molecular modeling, and DNA binding properties of a new series of imidazonaphthalimides. J. Med. Chem. 2002, 45, 5813–5816. [Google Scholar] [CrossRef]
- Braña, M.F.; Cacho, M.; Garcia, M.A. New Analogues of Amonafide and Elinafide, Containing Aromatic Heterocycles: Synthesis, Antitumor Activity, Molecular Modeling, and DNA Binding Properties. J. Med. Chem. 2004, 47, 1391–1399. [Google Scholar] [CrossRef]
- Antonini, I.; Polucci, P.; Magnano, A.; Sparapani, S.; Martelli, S. Rational design, synthesis, and biological evaluation of bis(pyrimido[5,6,1-de]acridines) and bis(pyrazolo[3,4,5-kl]acridine-5- carboxamides) as new anticancer agents. J. Med. Chem. 2004, 47, 5244–5250. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Q.; Qian, X. Novel heterocyclic family of phenyl naphthothiazole carboxamides derived from naphthalimides: Synthesis, antitumor evaluation, and DNA photocleavage. Bioorg. Med. Chem. 2005, 13, 3149–3155. [Google Scholar] [CrossRef]
- Houghton, P.J.; Cheshire, P.J.; Hallman, J.C., III; Gross, J.L.; McRipley, R.J.; Sun, J.H.; Behrens, C.H.; Dexter, D.L.; Houghton, J.A. Evaluation of a novel bis-naphthalimide anticancer agent, DMP 840, against human xenografts derived from adult, juvenile, and pediatric cancers. Cancer Chemother. Pharmacol. 1994, 33, 265–272. [Google Scholar]
- McRipley, R.J.; Burns-Horwitz, P.E.; Czerniak, P.M.; Diamond, R.J.; Diamond, M.A.; Miller, J.L.D.; Page, R.J.; Dexter, D.L.; Chen, S.-F.; Sun, J.-H.; et al. Efficacy of DMP 840: A novel bis-naphthalimide cytotoxic agent with human solid tumor xenograft selectivity. Cancer Res. 1994, 54, 159–164. [Google Scholar]
- Nitiss, J.L.; Zhou, J.; Rose, A.; Hsiung, Y.; Gale, K.C.; Osheroff, N. The bis(naphthalimide) DMP-840 causes cytotoxicity by its action against eukaryotic topoisomerase II. Biochemistry 1998, 37, 3078–3085. [Google Scholar] [CrossRef]
- Lai, C.-M.; Garner, D.M.; Gray, J.E.; Brogdon, B.L.; Peterman, V.C.; Pieniaszek H.J., Jr. Determination of bisnafide, a novel bis-naphthalimide anticancer agent, in human plasma by high-performance liquid chromatography with UV detection. J. Pharm. Biomed. Anal. 1998, 17, 427–434. [Google Scholar] [CrossRef]
- Bailly, C.; Braña, M.; Waring, M.J. Sequence-selective intercalation of antitumour bis-naphthalimides into DNA—Evidence for an approach via the major groove. Eur. J. Biochem. 1996, 240, 195–208. [Google Scholar] [CrossRef]
- Gallego, J.; Reid, B.R. Solution structure and dynamics of a complex between DNA and the antitumor bisnaphthalimide LU-79553: Intercalated ring flipping on the millisecond time scale. Biochemistry 1999, 38, 15104–15115. [Google Scholar] [CrossRef]
- Bousquet, P.F.; Braña, M.F.; Conlon, D.; Fitzgerald, K.M.; Perron, D.; Cocchiaro, C.; Miller, R.; Moran, M.; George, J.; Qian, X.D.; et al. Preclinical evaluation of LU 79553: A novel bis-naphthalimide with potent antitumor activity. Cancer Res. 1995, 55, 1176–1180. [Google Scholar]
- Villalona-Calero, M.A.; Eder, J.P.; Toppmeyer, D.L.; Allen, L.F.; Fram, R.; Velagapudi, R.; Myers, M.; Amato, A.; Kagen-Hallet, K.; Razvillas, B.; et al. Phase I and pharmacokinetic study of LU79553, a DNA intercalating bisnaphthalimide, in patients with solid malignancies. J. Clin. Oncol. 2001, 19, 857–869. [Google Scholar]
- Bailly, C.; Carrasco, C.; Joubert, A.; Bal, C.; Wattez, N.; Hildebrand, M.-P.; Lansiaux, A.; Colson, P.; Houssier, C.; Cacho, M.; et al. Chromophore-modified bisnaphthalimides: DNA recognition, topoisomerase inhibition, and cytotoxic properties of two mono- and bisfuronaphthalimides. Biochemistry 2003, 42, 4136–4150. [Google Scholar]
- Braña, M.F.; Cacho, M.; Ramos, A.; Dominguez, M.T.; Pozuelo, J.M.; Abradelo, C.; Rey-Stolle, M.F.; Yuste, M.; Carrasco, C.; Bailly, C. Synthesis, biological evaluation and DNA binding properties of novel mono and bisnaphthalimides. Org. Biomol. Chem. 2003, 1, 648–654. [Google Scholar] [CrossRef]
- Pavlov, V.; Lin, P.K.T.; Rodilla, V. Cytotoxicity, DNA binding and localisation of novel bis-naphthalimidopropyl polyamine derivatives. Chem. Biol. Interact. 2001, 137, 15–24. [Google Scholar] [CrossRef]
- Lin, P.K.T.; Pavlov, V.A. The synthesis and in vitro cytotoxic studies of novel bis-naphthalimidopropyl polyamine derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 1609–1612. [Google Scholar] [CrossRef]
- Lin, P.K.T.; Dance, A.M.; Bestwick, C.; Milne, L. The biological activities of new polyamine derivatives as potential therapeutic agents. Biochem. Soc. Trans. 2003, 31, 407–410. [Google Scholar] [CrossRef]
- Dance, A.-M.; Ralton, L.; Fuller, Z.; Milne, L.; Duthie, S.; Bestwick, C.S.; Lin, P.K.T. Synthesis and biological activities of bisnaphthalimido polyamines derivatives: Cytotoxicity, DNA binding, DNA damage and drug localization in breast cancer MCF 7 cells. Biochem. Pharmacol. 2005, 69, 19–27. [Google Scholar]
- Barrett, D.M.Y.; Kahwa, I.A.; Mague, J.T.; McPherson, G.L. Preparations, crystal structures, and unusual proton NMR characteristics of some phthalimides. J. Org. Chem. 1995, 60, 5946–5953. [Google Scholar] [CrossRef]
- Howell, R.C.; Edwards, S.H.; Gajadhar-Plummer, A.S.; Kahwa, I.A.; McPherson, G.L.; Mague, J.T.; White, A.J.P.; Williams, D.J. Phthalimides: Supramolecular interactions in crystals, hypersensitive solution 1H-NMR dynamics and energy transfer to europium(III) and terbium(III) states. Molecules 2003, 8, 565–592. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Werner, J.; Borowiak, T.; Wolska, I. Polyamines. I. Spectroscopic properties of N,N-bis-(phthalimidopropyl)-N-propylamine and supramolecular interactions in its crystals. J. Mol. Struct. 2006, 791, 137–143. [Google Scholar]
- Brycki, B.; Werner, J.; Kowalczyk, I.; Borowiak, T.; Wolska, I. Polyamines. III. Spectroscopic properties of N,N-bis-(phthalimidopropyl)-N-octylamine and supramolecular interactions in its crystals. J. Mol. Struct. 2010, 967, 34–41. [Google Scholar]
- Borowiak, T.; Wolska, I.; Brycki, B.; Grzesiak, W. Polyamines. IV. Spectroscopic properties of N,N-bis-(1,8-naphthalenedicarboximidopropyl)-N-propylamine and supramolecular interactions in its crystals. J. Mol. Struct. 2010, 979, 165–171. [Google Scholar]
- Matsubayashi, K.; Shiratori, H.; Kubo, Y. Effect of addition of trifluoroacetic acid on the photophysical properties and photoreactions of aromatic imides. Tetrahedron 2010, 66, 9291–9296. [Google Scholar] [CrossRef]
- Barooah, N.; Tamuly, C.; Baruah, J.B. Synthesis, characterisation of few N-substituted 1,8-naphthalimide derivatives and their copper(II) complexes. J. Chem. Sci. 2005, 117, 117–122. [Google Scholar]
- Kovalevsky, A.Y.; Ponomarev, I.I.; Antipin, M.Y.; Ermolenko, I.G.; Shishkin, O.V. Influence of steric and electronic effects of substituents on the molecular structures and conformational flexibility of 1,8-naphthalenedicarboximides. Russ. Chem. Bull. 2000, 49, 70–76. [Google Scholar]
- Sample Availability: Samples of the compounds NP2–NP9 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grzesiak, W.; Brycki, B. Synthesis, FTIR, 13C-NMR and Temperature-Dependent 1H‑NMR Characteristics of Bis-naphthalimide Derivatives. Molecules 2012, 17, 12427-12448. https://doi.org/10.3390/molecules171012427
Grzesiak W, Brycki B. Synthesis, FTIR, 13C-NMR and Temperature-Dependent 1H‑NMR Characteristics of Bis-naphthalimide Derivatives. Molecules. 2012; 17(10):12427-12448. https://doi.org/10.3390/molecules171012427
Chicago/Turabian StyleGrzesiak, Waldemar, and Bogumił Brycki. 2012. "Synthesis, FTIR, 13C-NMR and Temperature-Dependent 1H‑NMR Characteristics of Bis-naphthalimide Derivatives" Molecules 17, no. 10: 12427-12448. https://doi.org/10.3390/molecules171012427
APA StyleGrzesiak, W., & Brycki, B. (2012). Synthesis, FTIR, 13C-NMR and Temperature-Dependent 1H‑NMR Characteristics of Bis-naphthalimide Derivatives. Molecules, 17(10), 12427-12448. https://doi.org/10.3390/molecules171012427