An Efficient Synthesis of Novel Dispirooxindole Derivatives via One-Pot Three-Component 1,3-Dipolar Cycloaddition Reactions
Abstract
:1. Introduction
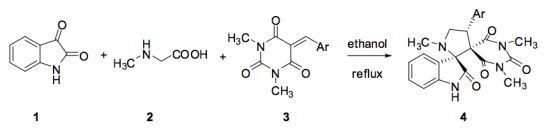
2. Results and Discussion

| Entry | Solvent | Time (h) | Yield b (%) |
|---|---|---|---|
| 1 | Ethanol | 2 | 84 |
| 2 | Methanol | 2 | 56 |
| 3 | Acetonitrile | 3 | 75 |
| 4 | Tetrahydrofuran (THF) | 6 | 80 |
| 5 | 1,4-Dioxane | 8 | 60 |
| Entry | Product | Ar | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 4a | 4-BrC6H4 | 2 | 84 |
| 2 | 4b | 4-CH3C6H4 | 2.5 | 75 |
| 3 | 4c | 4-NO2C6H4 | 1.5 | 90 |
| 4 | 4d | 4-ClC6H4 | 2 | 88 |
| Entry | Product | Ar | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 6a | 4-BrC6H4 | 1 | 84 |
| 2 | 6b | 4-CH3C6H4 | 2 | 81 |
| 3 | 6c | 4-NO2C6H4 | 1 | 87 |
| 4 | 6d | 4-ClC6H4 | 1 | 83 |
| 5 | 6e | C6H5 | 1 | 82 |
| 6 | 6f | 2-NO2C6H4 | 1.5 | 82 |
| 7 | 6g | 3,4-Cl2C6H3 | 1.5 | 88 |
| 8 | 6h | Thiophen-2-yl | 3 | 86 |

| Entry | Product | Ar | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 8a | C6H5 | 1 | 82 |
| 2 | 8b | 3,4-Cl3C6H3 | 1.5 | 80 |
| 3 | 8c | 3,4-OCH2OC6H3 | 3 | 78 |


3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Dispirooxindoles 4, 6 and 8
3.3. X-ray Crystallography [46]
4. Conclusions
Acknowledgments
References and Notes
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. Engl. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaymé, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Dou, G.L.; Shi, C.L.; Shi, D.Q. Highly Regioselective Synthesis of Polysubstituted Pyrroles through Three-Component Reaction Induced by Low-Valent Titanium Reagent. J. Comb. Chem. 2008, 10, 810–813. [Google Scholar] [CrossRef]
- Dondas, H.A.; Fishwick, C.W.G.; Grigg, R.; Kilner, C. 1,3-Dipolar cycloaddition of stabilised and non-stabilised azomethine ylides derived from uracil polyoxin C (UPoC): Access to nikkomycin analogues. Tetrahedron 2004, 60, 3473–3485. [Google Scholar] [CrossRef]
- Boruah, M.; Konwar, D.; Sharma, S.D. KF/Al2O3 mediated 1,3-dipolar cycloaddition of azomethine ylides: A novel and convenient procedure for the synthesis of highly substituted pyrrolidines. Tetrahedron Lett. 2007, 48, 4535–4537. [Google Scholar] [CrossRef]
- Kawashima, K.; Kakehi, A.; Noguchi, M. Generation of functionalized azomethine ylides and their application to stereoselective heterocycle synthesis: An equivalent process of C-unsubstituted nitrile ylide cycloaddition reaction. Tetrahedron 2007, 63, 1630–1643. [Google Scholar] [CrossRef]
- Watson, A.A.; Flett, G.W.J.; Asano, N.; Molyneux, R.J.; Nash, R.J. Polyhydroxylated alkaloids-natural occurrence and therapeutic applications. Phytochemistry 2001, 56, 265–295. [Google Scholar]
- Horri, S.; Fukase, H.; Matsuo, T.; Kameda, Y.; Asano, N.; Matsui, K. Synthesis and alpha-D-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J. Med. Chem. 1986, 29, 1038–1046. [Google Scholar] [CrossRef]
- Spearman, M.A.; Jamieson, J.C.; Wright, J.A. Studies on the effect of glycoprotein processing inhibitors on fusion of L6 nyoblast cell lines. Exp. Cell Res. 1987, 168, 116–126. [Google Scholar] [CrossRef]
- Karpas, A.; Fleet, G.W.J.; Dwek, R.A.; Petursson, S.; Namgoong, S.K.; Pamsden, N.G.; Jacob, G.S.; Rademacher, T.W. Aminosugar derivatives as potential anti-human immunodeficiency virus agents. Proc. Natl. Acad. Sci. USA 1988, 85, 9229–9233. [Google Scholar]
- Alcaide, B.; Almendros, P.; Alonso, J.M.; Aly, M.F. Rapid and Stereocontrolled Synthesis of Racemic and Optically Highly Functionalized Pyrrolidine Systems via Rearrangement of 1,3-Dipolar Cycloadducts Derived from 2-Azetidinone-Tethered Azomethine Ylides. J. Org. Chem. 2001, 66, 1351–1358. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tsuda, M.; Agemi, K.; Shigemori, H.; Ishibashi, M.; Sasaki, T.; Mikami, Y. Purealidines B and C, new bromotyrosine alkaloids from the okinawan marine sponge psammaplysilla purea. Tetrahedron 1991, 47, 6617–6622. [Google Scholar] [CrossRef]
- James, D.M.; Kunze, H.B.; Faulkner, D.J. Two New Brominated Tyrosine Derivatives from the Sponge Druinella (=Psammaplysilla) purpurea. J. Nat. Prod. 1991, 54, 1137–1140. [Google Scholar] [CrossRef]
- Dandia, A.; Singh, R.; Khaturia, S.; Merienne, C.; Morgant, G.; Loupy, A. Efficient microwave enhanced regioselective synthesis of a series of benzimidazolyl/triazolyl spiro[indole-thiazolidinones] as potent antifungal agents and crystal structure of spiro[3H-indole-3,2'-thiazolidine]-3'(1,2,4-triazol-3-yl)-2,4'(1H)-dione. Bioorg. Med. Chem. 2006, 14, 2409–2417. [Google Scholar] [CrossRef]
- Sebahar, P.R.; Williams, R.M. The Asymmetric Total Synthesis of (+)- and (−)-Spirotryprostatin B. J. Am. Chem. Soc. 2000, 122, 5666–5667. [Google Scholar] [CrossRef]
- Ma, J.; Hecht, S.M. Javaniside, a novel DNA cleavage agent from Alangium javanicum having a unusual oxindole skeleton. Chem. Commun. 2004, 1190–1191. [Google Scholar]
- Kang, T.H.; Matsumoto, K.; Tohda, M.; Murakami, Y.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1 and 5-HT2 receptors expressed in Xenopus oocyte. Eur. J. Pharmacol. 2002, 444, 39–45. [Google Scholar] [CrossRef]
- Ding, K.; Lu, Y.; Coleska, N.Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P.P.; Tomita, Y.; et al. Structure-Based Design of Potent Non-Peptide MDMZ Inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131. [Google Scholar]
- Abou-Gharbia, M.A.; Doukas, P.H. Synthesis of Tricyclic Arylspiro Compounds as Potential Antileukemic and Anticonvulsant Agents. Heterocycles 1979, 12, 637–640. [Google Scholar] [CrossRef]
- Kornet, M.J.; Thio, A.P. Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem. 1976, 19, 892–898. [Google Scholar] [CrossRef]
- Thetford, D.; Chorlton, A.P.; Hardman, J. Synthesis and properties of some polycyclic barbiturate pigments. Dyes Pigments 2003, 59, 185–191. [Google Scholar] [CrossRef]
- Bartzatt, R. Determination of barbituric acid, utilizing a rapid and simple colorimetric assay. J. Pharm. Biomed. Anal. 2002, 29, 909–915. [Google Scholar] [CrossRef]
- Andreu, R.; Garin, J.; Orduna, J.; Alcala, R.; Villacampa, B. Novel NLO-phores with Proaromatic Donor and Acceptor Groups. Org. Lett. 2003, 5, 3143–3146. [Google Scholar] [CrossRef]
- Meusel, M.; Ambrozak, A.; Hecker, T.K.; Gutschow, M. The Aminobarbituric Acid-Hydrantoin Rearrangement. J. Org. Chem. 2003, 68, 4684–4692. [Google Scholar] [CrossRef]
- Brunner, H.; Ittner, K.P.; Lunz, D.; Schmatloch, S.; Schmidt, T.; Zabel, M. Highly Enriched Mixtures of Methohexital Stereoisomers by Palladium-Catalyzed Allylation and Their Anaesthetic Activity. Eur. J. Org. Chem. 2003, 5, 855–862. [Google Scholar]
- Neumann, D.M.; Jursic, B.S.; Martin, K.L. Preparation of 5-(cyclohexylmethyl)barbituric acid derivatives by acid-catalyzed reductive cyclohexylmethylation of barbituric acids with p-hydroxy or p-methoxybenzaldehydes. Tetrahedron Lett. 2002, 43, 1603–1606. [Google Scholar] [CrossRef]
- Jursic, B.S.; Neumann, D.M. Preparation of 5-formyl- and 5-acetylbarbituric acids, including the corresponding Schiff bases and phenylhydrazones. Tetrahedron Lett. 2001, 42, 8435–8439. [Google Scholar] [CrossRef]
- Galati, E.M.; Monforte, M.T.; Miceli, N.; Raneri, E. Anticonvulsant and sedative effects of some 5-substituted bromopyrazolinic spirobarbiturates. Farmaco 2001, 56, 459–461. [Google Scholar] [CrossRef]
- Singh, P.; Paul, K. A practical approach for spiro- and 5-monoalkylated barbituric acid. J. Heterocycl. Chem. 2006, 43, 607–612. [Google Scholar] [CrossRef]
- Lomlm, L.; Einsiedel, J.; Heinemann, F.W.; Meyer, K.; Gmeiner, P. Proline Derived Spirobarbiturates as Highly Effective β-Turn Mimetics Incorporating Polar and Functionalizable Constraint Elements. J. Org. Chem. 2008, 73, 3608–3611. [Google Scholar] [CrossRef]
- Liu, H.; Dou, G.L.; Shi, D.Q. Regio- and Stereoselective Synthesis of Novel Spiropyrrolidine Bioxindole Derivatives via Multicomponent Reactions. J. Comb. Chem. 2010, 12, 292–294. [Google Scholar] [CrossRef]
- Liu, H.; Dou, G.L.; Shi, D.Q. Regioselective Synthesis of Novel Spiropyrrolidines and Spirothiapyrrolizidines Through Multicomponent 1,3-Dipolar Cycloaddition Reaction of Azomethin Ylides. J. Comb. Chem. 2010, 12, 633–637. [Google Scholar] [CrossRef]
- Chen, H.; Shi, D.Q. Efficient one-pot synthesis of spiro[indoline-3,4'-pyrazolo[3,4-e][1,4]thiazepine]dione via three-component reaction. Tetrahedron 2011, 67, 5686–5692. [Google Scholar] [CrossRef]
- Hu, Y.; Zou, Y.; Wu, H.; Shi, D.Q. A facile and efficient ultrasound-assisted synthesis of novel dispiroheterocycles through 1,3-dipolar cycloaddition reactions. Ultrason. Sonochem. 2012, 19, 264–269. [Google Scholar] [CrossRef]
- Liu, H.; Zou, Y.; Hu, Y.; Shi, D.Q. An Efficient One-Pot Synthesis of Dispiropyrrolidine Derivatives Through 1,3-Dipolar Cycloaddition Reactions Under Ultrasound Irradiation. J. Heterocycl. Chem. 2011, 48, 877–881. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- We have calculated possible configurations of the products at the DFT level of theory, and all the calculations were performed at the B3LYP/6-31G level of theory. First, the geometrical optimizations of the two possible configurations were obtained, and then the lowest energy minimum corresponds to the configurations were calculated. All calculations were carried out with a Gaussian 09W program package.
- Ardill, H.; Dorrity, M.J.R.; Grigg, R.; Leon-Ling, M.S.; Malone, J.F.; Sridharan, V.; Thianpatanagul, S. X=Y-ZH compounds as potential 1,3-Dipoles. Part 28, the iminium ion route to azomethine ylides. Background and reaction of amines with bifunctional ketones. Tetrahedron 1990, 46, 6433–6448. [Google Scholar] [CrossRef]
- Ardill, H.; Fontaine, X.L.R.; Grigg, R.; Henderson, D.; Montgomery, J.; Sridharan, V.; Surendrakumar, S. X=Y-ZH compounds as potential 1,3-dipoles. Part 29. The iminium ion route to azomethine ylides. Reaction of cyclic secondary amines with mono- and bi-functional aldehydes. Tetrahedron 1990, 46, 6449–6466. [Google Scholar]
- Subramaniyan, G.; Raghunathan, R.; Nethaji, M. A facile entry into a new class of spiroheterocycles: Synthesis of dispiro[oxindolechromanone/flavanone/tetralone]pyrroloiso-quinoline ring systems. Tetrahedron 2002, 58, 9075–9079. [Google Scholar] [CrossRef]
- Pardasani, R.T.; Pardasani, P.; Chaturvedi, V.; Yadav, S.K.; Saxena, A.; Sharma, I. Theoretical and synthetic approach to novel spiroheterocycles derived from isatin derivatives and L-proline via 1,3-dipolar cycloaddition. Heteroat. Chem. 2003, 14, 36–41. [Google Scholar] [CrossRef]
- Lakshmi, N.V.; Thirumurugan, P.; Perumal, P.T. An expedient approach for the synthesis of dispiropyrrolidine bisoxindoles, spiropyrrolidine oxindoles and spiroindane-1,3-diones through 1,3-dipolar cycloaddition reactions. Tetrahedron Lett. 2010, 51, 1064–1068. [Google Scholar] [CrossRef]
- Jursic, B.S.; Neumann, D.M. Reductive C-alkylation of barbituric acid derivatives with carbonyl compounds in the presence of platinum and palladium catalysts. Tetrahedron Lett. 2001, 42, 4103–4107. [Google Scholar] [CrossRef]
- Krasnov, K.A.; Kartsev, V.G.; Gorovoi, A.S.; Khrustalev, V.N. Chemical Modification of Plant Alkaloids. III. X-Ray Diffraction and NMR Studies of the Structure of 1,3-Dimethyl-5-arylmethyl-5-cytisylmethylbarbituric Acids. Chem. Nat. Compd. 2002, 38, 450–457. [Google Scholar] [CrossRef]
- Crystallographic data for 8b have been deposited at the Cambridge Crystallographic Data Centre with the deposition number CCDC906792. Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB 2 1EZ, UK; Fax: +44-1223-336-033; or Email: [email protected]).
- Sample Availability: Samples of the compounds 4 and 6 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, Z.; Zhao, Q.; Chen, G.; Wang, H.; Lin, W.; Xu, L.; Liu, H.; Wang, J.; Shi, D.; Wang, Y. An Efficient Synthesis of Novel Dispirooxindole Derivatives via One-Pot Three-Component 1,3-Dipolar Cycloaddition Reactions. Molecules 2012, 17, 12704-12717. https://doi.org/10.3390/molecules171112704
Huang Z, Zhao Q, Chen G, Wang H, Lin W, Xu L, Liu H, Wang J, Shi D, Wang Y. An Efficient Synthesis of Novel Dispirooxindole Derivatives via One-Pot Three-Component 1,3-Dipolar Cycloaddition Reactions. Molecules. 2012; 17(11):12704-12717. https://doi.org/10.3390/molecules171112704
Chicago/Turabian StyleHuang, Zhibin, Qian Zhao, Gang Chen, Huiyuan Wang, Wei Lin, Lexing Xu, Hongtao Liu, Juxian Wang, Daqing Shi, and Yucheng Wang. 2012. "An Efficient Synthesis of Novel Dispirooxindole Derivatives via One-Pot Three-Component 1,3-Dipolar Cycloaddition Reactions" Molecules 17, no. 11: 12704-12717. https://doi.org/10.3390/molecules171112704
APA StyleHuang, Z., Zhao, Q., Chen, G., Wang, H., Lin, W., Xu, L., Liu, H., Wang, J., Shi, D., & Wang, Y. (2012). An Efficient Synthesis of Novel Dispirooxindole Derivatives via One-Pot Three-Component 1,3-Dipolar Cycloaddition Reactions. Molecules, 17(11), 12704-12717. https://doi.org/10.3390/molecules171112704