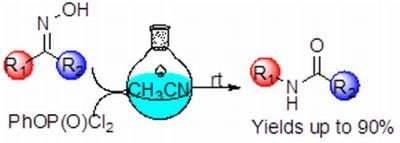
Beckmann Rearrangement of Ketoximes Induced by Phenyl Dichlorophosphate at Ambient Temperature
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. General Procedure for the Synthesis of Compounds in Table 2
3.3. Gram-Scale Synthesis of Amide 2
4. Conclusions
Acknowledgments
References
- Sato, O.; Ikushima, Y.; Yokoyama, T. Noncatalytic Beckmann rearrangement of cyclohexanone-oxime in supercritical water. J. Org. Chem. 1998, 63, 9100–9102. [Google Scholar] [CrossRef]
- Li, D.; Shi, F.; Guo, S.; Deng, Y. Highly efficient Beckmann rearrangement and dehydration of oximes. Tetrahedron Lett. 2005, 46, 671–674. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Gopalaiah, K. Beckmann reaction of oximes catalyzed by chloral: Mild and neutral procedures. Tetrahedron Lett. 2003, 44, 755–756. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Gopalaiah, K. Ketones to amides via a formal Beckmann rearrangement in “one pot”: A solvent-free reaction promoted by anhydrous oxalic acid. Possible analogy with the Schmidt reaction. Tetrahedron Lett. 2003, 44, 7437–7439. [Google Scholar] [CrossRef]
- Gawly, R.E. The Beckmann reaction: Rearrangements, elimination-additions, fragmentations, and rearrangement-cyclization. Org. React. 1988, 35, 14–24. [Google Scholar]
- Smith, M.B.; March, J. Rearrangements. In Advanced Organic Chemistry, 5th ed.; John Wiley & Sons: New York, NY, USA, 2001; p. 1415. [Google Scholar]
- Furuya, Y.; Ishihara, K.; Yamamoto, H. Cyanuric Chloride as a Mild and Active Beckmann Rearrangement Catalyst. J. Am. Chem. Soc. 2005, 127, 11240–11241. [Google Scholar] [CrossRef] [PubMed]
- Luca, L.D.; Giacomelli, G.; Porcheddu, A. Beckmann rearrangement of oximes under very mild conditions. J. Org. Chem. 2002, 67, 6272–6274. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Obora, Y.; Sakaguchi, S.; Ishii, Y. Beckmann rearrangement of ketoximes to lactams by triphosphazene catalyst. J. Org. Chem. 2008, 73, 2894–2897. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.F.; Xia, C.G.; Chen, J. p-Toluenesulfonic acid mediated zinc chloride: Highly effective catalyst for the Beckmann rearrangement. Tetrahedron Lett. 2007, 48, 7218–7221. [Google Scholar] [CrossRef]
- Zhu, M.; Cha, C.; Deng, W.P.; Shi, X.X. A mild and efficient catalyst for the Beckmann rearrangement, BOP-Cl. Tetrahedron Lett. 2006, 47, 4861–4863. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.; Luo, C.; Yang, T.; Yang, L.; Suo, J. Sulfamic acid as a cost-effective and recyclable catalyst for liquid Beckmann rearrangement, a green process to produce amides from ketoximes without waste. Tetrahedron Lett. 2004, 45, 3369–3372. [Google Scholar] [CrossRef]
- Sardarian, A.R.; Shahsavari-Fard, Z.; Shahsavari, H.R.; Ebrahimi, Z. Efficient Beckmann rearrangement and dehydration of oximes via phosphonate intermediates. Tetrahedron Lett. 2007, 48, 2639–2643. [Google Scholar] [CrossRef]
- Gui, J.; Deng, Y.; Hu, Z.; Sun, Z. A novel task-specific ionic liquid for Beckmann rearrangement: A simple and effective way for product separation. Tetrahedron Lett. 2004, 45, 2681–2683. [Google Scholar] [CrossRef]
- Ramalingan, C.; Park, Y.T. Mercury-catalyzed rearrangement of ketoximes into amides and lactams in acetonitrile. J. Org. Chem. 2007, 72, 4536–4538. [Google Scholar] [CrossRef] [PubMed]
- Owston, N.A.; Parker, A.J.; Williams, J.M. Highly efficient ruthenium-catalyzed oxime to amide rearrangement. Org. Lett. 2007, 9, 3599–3601. [Google Scholar] [CrossRef] [PubMed]
- Yadav, L.D.S.; Patel, R.; Srivastava, V.P. Bromodimethylsulfonium bromide-ZnCl2: A mild and efficient catalytic system for Beckmann rearrangement. Synthesis 2010, 6, 1771–1776. [Google Scholar] [CrossRef]
- Pi, H.J.; Dong, J.D.; An, N.; Du, W.; Deng, W.P. Unexpected results from the re-investigation of the Beckmann rearrangement of ketoximes into amides by using TsCl. Tetrahedron 2009, 65, 7790–7793. [Google Scholar] [CrossRef]
- Karimi, B.; Behzadnia, H. Novel periodic mesoporous silica chloride (PMSCl) with 2D P6mm hexagonal structures: Efficient catalysts for the Beckmann rearrangement. Synlett 2010, 13, 2019–2023. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Mondal, P. Efficient iodine-mediated Beckmann rearrangement of ketoximes to amides under mild neutral conditions. Synthesis 2010, 21, 3705–3709. [Google Scholar] [CrossRef]
- Srivastava, V.P.; Patel, R.; Yadav, L.D.S. Cyclopropenium ion catalyzed Beckmann rearrangement. Chem. Commun. 2010, 46, 5808–5810. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, Y.; Hatakeda, K.; Sato, O.; Yokoyama, T.; Arai, M. Acceleration of synthetic organic reactions using supercritical water: Noncatalytic Beckmann and Pinacol rearrangement. J. Am. Chem. Soc. 2000, 122, 1908–1918. [Google Scholar] [CrossRef]
- Boero, M.; Ikeshoji, T.; Liew, C.C.; Terakura, K.; Parrinello, M. Hydrogen bond driven chemical reactions: Beckmann rearrangement into ε-caprolactam in supercritical water. J. Am. Chem. Soc. 2004, 126, 6280–6286. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Chan, W.H.; Lee, S.P. Convenient procedure for esterification of carboxylic acids. Tetrahedron Lett. 1978, 19, 4461–4464. [Google Scholar]
- Liu, H.J.; Sabesan, S.I. Direct transformation of carboxylic acids to thiol esters induce by phenyl dichlorophosphate. Can. J. Chem. 1980, 58, 2645–2648. [Google Scholar] [CrossRef]
- Liu, H.J.; Lamoureux, G.V.; Brunet, M.L. Transformation of β-diketones to β-chloro-α,β-unsaturated ketones induced by lithium hydride and phenyl dichlorophosphate. Can. J. Chem. 1986, 64, 520–522. [Google Scholar] [CrossRef]
- Liu, H.J.; Nyangulu, J.M. Facile fragmentation of pienes using dimethyl sulfoxide activated by phenyl dichlorophosphate or phosphorus oxychloride. Efficient conversion of α-pinene to carvone. Tetrahedron Lett. 1989, 30, 5097–5098. [Google Scholar] [CrossRef]
- Liu, H.J.; Nyangulu, J.M. A facile method for thr synthesis of β-chloroalkyl sulfides using dimethyl sulfoxide activated by phenyl dichlorophosphate or phosphorus oxychloride. Tetrahedron Lett. 1988, 29, 5467–5470. [Google Scholar] [CrossRef]
- Liu, H.J.; Nyangulu, J.M. Oxidative deamination of benzylic amines induced by phenyl dichlorophosphate. Synth. Commun. 1989, 19, 3407–3411. [Google Scholar] [CrossRef]
- Liu, H.J.; Yu, S.Y. Deketalization induced by phenyl dichlorophosphate and sodium iodide. Synth. Commun. 1986, 16, 1357–1361. [Google Scholar] [CrossRef]
- Liu, H.J.; Wiszniewski, V. A new procedure for dethioacetalization. Tetrahedron Lett. 1988, 29, 5471–5474. [Google Scholar] [CrossRef]
- Kuo, C.W.; Zhu, J.L.; Wu, J.D.; Chu, C.M.; Yao, C.F.; Shia, K.S. A convenient new procedure for converting primary amides into nitriles. Chem. Commun. 2007, 2007, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Lee, F.Y.; Wu, J.D.; Kuo, C.W.; Shia, K.S. An efficient new procedure for the one-pot conversion of aldehydes into the corresponding nitriles. Synlett 2007, 2007, 1317–1319. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Z. Silica-supported dichlorophosphate catalyzed Beckmann rearrangement and dehydration of oximes under microwave irradiation. Lett. Org. Chem. 2008, 5, 495–501. [Google Scholar] [CrossRef]
- Iranpoor, N.; Firouzabadi, H.; Jamalian, A.; Tamami, M. Silphos [PCl3-n(SiO2)n], a heterogeneous phosphine reagent mediated the conversion of oximes to nitriles and amides or carbonyl compounds. Lett. Org. Chem. 2006, 3, 267–270. [Google Scholar] [CrossRef]
- Imamoto, T.; Yokoyama, H.; Yokoyama, M. Trimethylsilyl polyphosphate (PPSE), a useful reagent for the Beckmann rearrangement. Tetrahedron Lett. 1981, 22, 1803–1804. [Google Scholar] [CrossRef]
- Bosch, A.I.; Cruz, P.; Diez-Barra, E.; Loupy, A.; Langa, F. Microwave assisted Beckmann rearrangement of ketoximes in dry media. Synlett 1995, 1995, 1259–1260. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Wu, P.; Tatsumi, T. Selective production of e-caprolactam via liquid-phase Beckmann rearrangement of cyclohexanone oxime over HUSY catalyst. Chem. Lett. 2004, 33, 1288–1289. [Google Scholar] [CrossRef]
- Botella, P.; Corma, A.; Iborra, S.; Monton, R.; Rodriguez, I.; Costa, V. Nanosized and delayered zeolitic materials for the liquid-phase Beckmann rearrangement of cyclododecanone oxime. J. Catal. 2007, 250, 161–170. [Google Scholar] [CrossRef]
- Mitsudome, T.; Matsuno, T.; Sueoka, S.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Titanium cation-exchanged montmorillonite as an active heterogeneous catalyst for the Beckmann rearrangement under mild reaction conditions. Tetrahedron Lett. 2012, 53, 5211–5214. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds in Table 2 are available from the authors. |


| Entry | Reagent | Catalyst b | Solvent | Time (h) | Yield (%) c |
|---|---|---|---|---|---|
| 1 | 3 | − | CH3CN | 3 | 83 |
| 2 | 3 | − | THF | 3 | 40 |
| 3 | 3 | − | Toluene | 16 | 87 |
| 4 | 3 | − | CH3CN | 1 | 86 |
| 5 | 4 | − | CH2Cl2 | 6 | 76 |
| 6 | 4 | − | Toluene | 14 | 74 |
| 7 | 4 | − | CH3CN | 2 | 81 |
| 8 | 5 | − | CH2Cl2 | 20 | 72 |
| 9 | 5 | − | Toluene | 20 | 70 |
| 10 | 5 | − | CH3CN | 5 | 80 |
| 11 | 6 | − | CH2Cl2 | 24 | 60 |
| 12 | 6 | − | Toluene | 26 | 54 |
| 13 | 6 | − | CH3CN | 7 | 61 |
| 14 | 7 | − | CH2Cl2 | 30 | 55 |
| 15 | 7 | − | Toluene | 30 | 50 |
| 16 | 7 | − | CH3CN | 8 | 52 |
| 17 | 3 | ZnCl2 | CH2Cl2 | 1 | 85 |
| 18 | 3 | ZnCl2 | CH3CN | 1 | 81 |
| 19 | 3 | ZnI2 | CH2Cl2 | 0.6 | 84 |
| 20 | 3 | ZnI2 | CH3CN | 0.8 | 79 |
| 21 | 3 | BCl3 | CH2Cl2 | 0.8 | 82 |
| 22 | 3 | BCl3 | CH3CN | 0.8 | 80 |
| 23 | 5 | ZnCl2 | CH2Cl2 | 5 | 79 |
| 24 | 5 | ZnI2 | CH2Cl2 | 3 | 83 |

  |
© 2012 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuo, C.-W.; Hsieh, M.-T.; Gao, S.; Shao, Y.-M.; Yao, C.-F.; Shia, K.-S. Beckmann Rearrangement of Ketoximes Induced by Phenyl Dichlorophosphate at Ambient Temperature. Molecules 2012, 17, 13662-13672. https://doi.org/10.3390/molecules171113662
Kuo C-W, Hsieh M-T, Gao S, Shao Y-M, Yao C-F, Shia K-S. Beckmann Rearrangement of Ketoximes Induced by Phenyl Dichlorophosphate at Ambient Temperature. Molecules. 2012; 17(11):13662-13672. https://doi.org/10.3390/molecules171113662
Chicago/Turabian StyleKuo, Chun-Wei, Min-Tsang Hsieh, Shijay Gao, Yi-Ming Shao, Ching-Fa Yao, and Kak-Shan Shia. 2012. "Beckmann Rearrangement of Ketoximes Induced by Phenyl Dichlorophosphate at Ambient Temperature" Molecules 17, no. 11: 13662-13672. https://doi.org/10.3390/molecules171113662
APA StyleKuo, C. -W., Hsieh, M. -T., Gao, S., Shao, Y. -M., Yao, C. -F., & Shia, K. -S. (2012). Beckmann Rearrangement of Ketoximes Induced by Phenyl Dichlorophosphate at Ambient Temperature. Molecules, 17(11), 13662-13672. https://doi.org/10.3390/molecules171113662